Chapter: Essentials of Psychiatry: Psychiatric Epidemiology
Types of Epidemiological Studies
Types of Epidemiological Studies
In general, epidemiological studies are designed to
find associa-tions between exposures and health outcomes. A main concern in
epidemiological studies is the selection of study groups on the basis of either
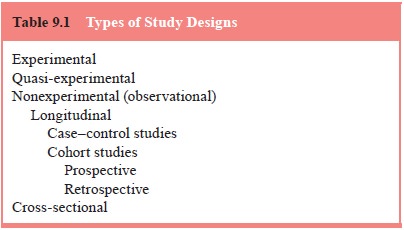
disease status or exposure status. Epidemiological studies (Table 9.1) can be
classified as 1) experimental, 2) quasi-experimental, and 3) nonexperimental or
observational.
Experimental Studies
The main distinction of experimental studies is that the investiga-tor assigns the status of exposure or nonexposure to each subject. The assignment to the exposure group becomes part of the study protocol. Once subjects are assigned to exposed or nonexposed groups, they are observed for a time, and observations about changes in morbidity are recorded. The most common experi-mental design is the clinical trial, in which clinical populations are exposed to a specific treatment protocol to measure an out-come, usually resolution of symptoms. To ensure the integrity of a clinical trial, three main elements are necessary (Miettinen, 1985): 1) “randomization”, to ensure comparability of the popu-lations; 2) “placebo”, to ensure comparability of the effects; and 3) “blinding”, to ensure comparability of information.
In randomization, subjects are randomly assigned to
dif-ferent exposure groups to attempt to ensure that subjects in each group
have similar clinical and demographic characteristics. Randomization should
theoretically achieve a balance of un-known factors in the different groups.
To control comparability of extraneous effects of a
specified treatment, experimental studies use placebo-controlled groups. A
placebo controls for factors that may affect the outcome of the study
independently of the exposure status. For example, if sub-jects in an open
trial are aware of what medication they receive, this knowledge could bias
their response to the treatment. Simi-larly, subjects who are aware of being in
an untreated control group could respond over time in a biased fashion. Thus,
one goal in as-signing patients at random to treatment or placebo-control
groups is to minimize observation bias. In a single-blind study, only the
patient is unaware of the actual treatment. In a double-blind study, the
investigator and the subject of investigation are unaware of treatment
assignment. In a triple-blind study, the data analyst is also not informed of
the meaning of the group assignment code.
Quasi-experimental Studies
Natural experiments that permit comparisons of two
populations, one that receives an exposure and the other that does not, are
re-ferred to as quasi-experimental studies. To be considered
quasi-experimental, baseline data must have been collected before the exposure
event. Without that requirement, the study is simply a retrospective
observational study
Nonexperimental Studies
Nonexperimental studies are divided into
cross-sectional and longitudinal designs
Cross-sectional Designs
Cross-sectional designs are typically employed in
surveys aimed at providing data on the distribution of disorders in the
popula-tion. Differences in rates by basic demographic data are also usu-ally
derived. In epidemiology, cross-sectional designs are usually best employed
when causal hypotheses are not being tested. For example, when a community
wants to investigate the distribu-tion of an illness to decide on the need for
psychiatric services, a cross-sectional survey is highly appropriate
Longitudinal Designs
Longitudinal designs are divided into case–control
and cohort studies and are characterized by a time interval between cause and
effect. In cross-sectional studies, there is no interval between exposure and
illness, which are measured at the same point in time.
Case–Control
Studies. In case–control studies, subjects are defined in terms of having (case patients) or not having
(controlpatients) the disease of interest. The groups are compared in terms of
history of exposure. In general, two types of control groups are used: hospital
control groups and population control groups. The selection of the control
group is a key point in terms of validity. Control subjects should be selected
independently of exposure status. Case and control patients may be matched on
different characteristics, the key issue being that control patients should
represent those individuals who, if they had the disease, would be selected as
case patients (Miettinen, 1985).
Case–control studies can assess whether a risk
factor is more prevalent in case than in control patients but may not be able
to establish the rate of disease after exposure to that risk factor. For the purpose
of estimating the true rate of disease as-sociated with an exposure, the
prospective cohort study design is the preferable methodology.
Cohort
Studies. In cohort studies, subjects are identified in terms of exposure or nonexposure status and are observed for a
specified time to determine the presence or absence of a health outcome. Cohort
studies are divided into prospective and retro-spective. In prospective cohort
studies, the exposure or nonex-posure status is defined when the study is
initiated. The subjects of investigation are followed up into the future to
determine dis-ease or nondisease status. In retrospective studies, the status
of exposed or nonexposed is defined in the present. In prospective cohort
studies, exposures of the present are evaluated; in retro-spective cohort
studies, exposures of the past are being evalu-ated. Cohort groups share the
common exposure status and are observed to ascertain the presence or absence of
a disease or outcome.
For comparison groups, a cohort study can use an
internal subset of the population under study, by comparing exposed with
unexposed members of the cohort, or an external comparison. A comparison cohort
can be selected from a similarly defined population.
The major strength of the cohort design is the
possibility of estimating a temporal relationship between exposure and
dis-ease. With a cohort study, it is possible to study rare exposures and to
evaluate multiple outcomes from a single exposure. The limitation of cohort
studies is primarily one of feasibility because most such studies are expensive
and involve study populations who are difficult to recruit and maintain for
follow-up
Related Topics