Chapter: Essentials of Psychiatry: Stimulants and Related Compounds
Treatment with Stimulant Medications
Treatment
with Stimulant Medications
An
effective treatment strategy for this chronic disorder of ADHD requires a plan
for follow-up and monitoring. Techniques involve regular follow-up visits, the
use of rating forms from parent and teacher, and the monitoring of academic
progress in the school. Seeing the patient and a family member regularly is
essential, often on a once-monthly basis when the medication prescription must
be renewed.
Preparation of the Patient
Does the child’s attitude about drug treatment influence its success? Concerns that medications may create negative attributions or dysphoric effects in children with ADHD have not as a rule been confirmed in direct tests. Excellent studies show that there is considerable variability in children’s attributions, as well as their mood response to pills. Pelham and colleagues (1992) found a subgroup of “depressogenic” children who gave external credit for positive events and internal blame for negative events. This group was above the median on attributions of success to their pills. Comorbidity and family history for anxiety and/or depression seemingly are relevant variables in prediction of such attributional effects. During treatment, a small percentage of stimulant-treated children do become dysphoric, weepy and mournful. Children who respond with depressogenic attributions or dysphoria may be children whose depressive diathesis is indistinguishable from ADHD and who are therefore mistakenly diagnosed as ADHD and treated with stimulants. Given the overlap of depression and ADHD, and the findings that depressogenic cognitive styles predict later depression, it could be important to identify those children who may be prone to attribute their success to pills rather than to their own effort, even though most patients do not do so.
Physicians
can explain the reasons for taking pills and how the pills can prove helpful in
school and at home. Children often have negative attitudes about treatment,
thinking of treatment as a punishment. Pill taking can be socially stigmatizing
to the child with ADHD if the daily trip to the nurse generates peer ridicule.
Discussing the problem of in-school pill administration may be of great
interest to the child, and he or she should be included in all discussions
about dosing and changing of doses. At best, it may help with compliance.
Consideration of Side Effects
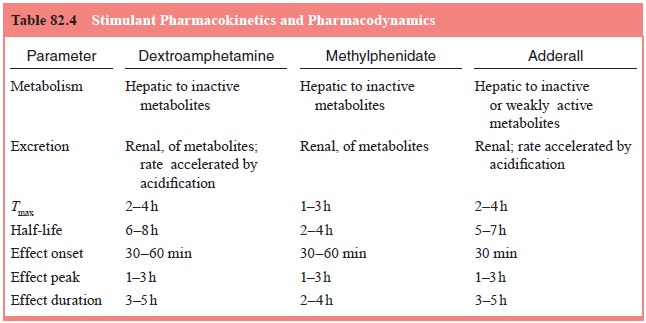
Common
side effects and their management are noted in Table 82.4. Stimulant side
effects are dose dependent and range from mild to moderate in most children.
The management of stimulant-related adverse effects generally involves a
temporary reduction of dose or a change in time of dosing. MPH has an excellent
safety record, probably because the duration of action is so brief.
Rebound One adverse effect, commonly
known as behavioral rebound, appears
when children experience psychostimulant withdrawal at the end of the school
day. Children present with afternoon irritability, overtalkativeness,
noncompliance, excit-ability, motor hyperactivity and insomnia about 5 to 15
hours after the last dose. If rebound occurs, many physicians add a small
afternoon MPH dose or add a small dose of a tricyclic antidepressant.
Seizure Disorders A
commonly held notion is that stimulants lower
the seizure threshold. The PDR warns against the use of stimulants in children
with preexisting seizure disorders. Both ADHD and seizure disorders are quite
prevalent and may occur in the same individual, so the question of the risk of
stimulant treatment may frequently arise. Current practice is to give chil-dren
with ADHD and epilepsy a combination of anticonvulsant and MPH. Plasma levels
of the anticonvulsant should be moni-tored to avoid toxicity resulting from
MPH’s competitive inhibi-tion of metabolic pathways. At the doses used to treat
ADHD in children, the psychostimulants have variable but minimal effects on the
seizure threshold.
Long-term Stimulant Effects: Growth Velocity Reductions
Adults
taking amphetamines routinely experience weight loss, which can be attributed
to reduced appetite and decreased food intake. The drug’s action on the lateral
hypothalamic feeding center may explain this effect. Appetite suppression
differs among different species, with humans showing only mild effects with
rapid onset of tolerance. Children with ADHD taking psy-chostimulants routinely
show appetite suppression when starting treatment. For this reason, dosing
should optimally occur after breakfast and lunch. Even though the daytime
appetite is re-duced, hunger rebounds in the evening. These effects on appetite
often decrease within the first 6 weeks of treatment.
The
growth effects of MPH appear to be minimal. Height and weight should be
measured at 6-month intervals during stim-ulant treatment and recorded on
age-adjusted growth forms to determine the presence of a drug-related reduction
in height or weight velocity. If such a decrement is discovered during
main-tenance therapy with psychostimulants, a reduction in dosage or change to
another class of medication can be carried out.
Continuing Stimulant Treatment in Patients with Tic Disorders
The PDR
suggests that the presence of tics is a contraindication to stimulant usage.
With the comorbidity of ADHD in children with Tourette’s disorder estimated to
fall between 20 and 54%, there is a concern that a stimulant’s dopamine agonist
action will unmask, trigger, accelerate, or provoke irreversible Tourette’s
symptoms. This worry is enhanced by a number of papers, some of them
retrospective, that reported onset of tics after stimulant treatment had begun.
Drug Interactions
(See
Table 82.5 for a listing of common drug interactions.) Mixing psychostimulants
with other psychotropic medications is generally not advisable. Most serious is
the addition of a psy-chostimulant to a monoamine oxidase inhibitor
antidepressant regimen, a potentially lethal combination that can elevate blood
pressure to dangerous levels. Additive effects between psychos-timulants and
the systemic agents used to treat asthma can pro-duce feelings of dizziness,
tachycardia, palpitation, weakness and agitation. Theophylline, for example,
when taken orally (Theo-Dur Sprinkle), can have this undesirable agitating
effect. It is best to ask the pediatrician or allergist to switch from the orally
ingested preparation to an inhalant to avoid such additive sympathomimetic
effects.
Psychostimulants
compete with other medications for the same metabolic pathway and have been
thought to produce an increase in the plasma concentration of both drugs. The
psychostimulants can also interfere with the action of other medications. MPH
can block the antihypertensive action of guanethidine. DEX blocks the action of
some beta-adrenergic antagonists (e.g., propranolol) and slows the intestinal
absorption of phenytoin and phenobarbital. The renal clearance of DEX is
enhanced by urine acidifying agents, so that grapefruit juice will shorten the
elimination half-life of the medication. Conversely, urine alkalinizing agents
decrease clearance. This is also true for ritalinic acid, although this
metabolite is not active in the CNS. MPH may elevate the plasma level of
antidepressants, anticonvulsants, coumarin anticoagulants and phenylbutazone.
MPH may increase the concentration of the serotonin reuptake blocker fluoxetine,
potentially enhancing agitation, although no such side effect was seen in a
single case report of the treatment of an 11-year-old boy with both ADHD and
obsessive–compulsive disorder. Other medications have been combined
successfully with MPH, such as clonazepam to reduce tics. Clonidine has been
added to reduce sleep disturbances associated with ADHD and with stimulant
medication. Bupropion may exacerbate tics when added to MPH given to children
with ADHD.
Initiation of Treatment
Medication
treatment begins with a choice of medication. Although the MPH or DEX
psychostimulant types have equal efficacy, MPH is often used first.
Titration
serves two purposes: acclimatization of the child to the drug and determination
of his or her best dose. School-age children should be started with low doses
to minimize adverse effects. Psychostimulant medication should be taken at or
just after mealtime to lessen the anorectic effects; studies have shown
that food
may enhance drug absorption. MPH treatment can be initiated with a single 5 mg
dose at 8:00 am for 3 days; then a 5 mg dose at 8:00 am and at noon for the
next 3 days; then 10 mg at 8:00 am and 5 mg at noon for 3 days; and finally 10
mg at 8:00 am and 10 mg at noon are given and maintained for at least 2 weeks.
Preschoolers may start as low as 2.5 mg at 8:00 am but build to the same total
20 mg/day dose of MPH. The dosing instructions should be written down for the
parent, with dates and times specified in detail. A photocopy of the instructions
should be kept in the patient’s chart.
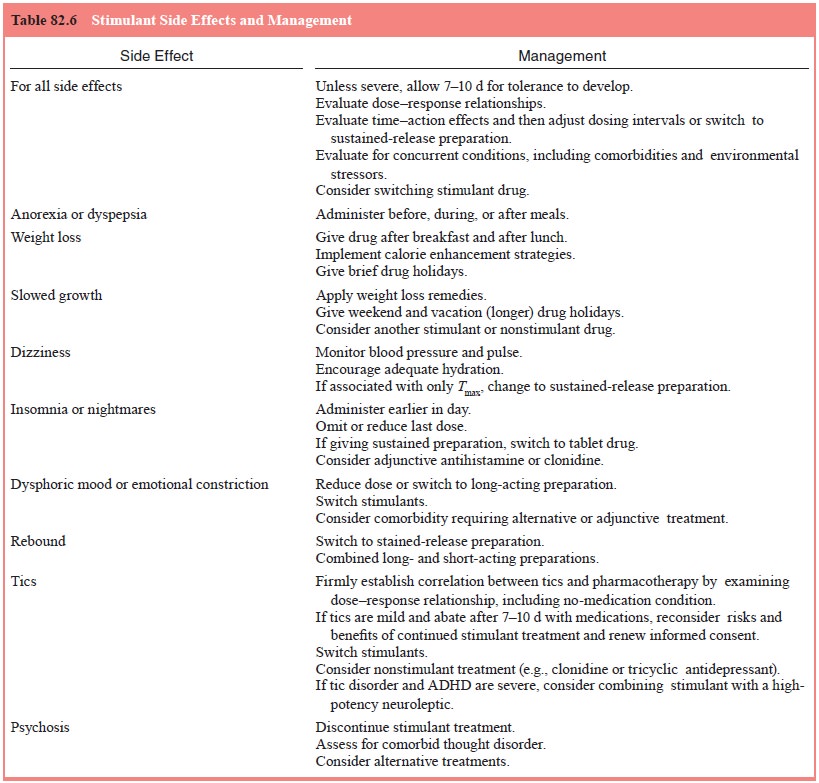
Table
82.6 is an inventory of stimulant drugs and doses. DEX is usually started at
2.5 to 5 mg/day and gradually increased in 2.5- to 5-mg increments; MPH is
usually started at 5 mg b.i.d. and then titrated in increments of 5 mg/dose
every 4 to 7 days. Peak behavioral effects are noted 1 to 3 hours after
ingestion and dissipate in 3 to 6 hours for both MPH and DEX.
The
Conners Teacher Rating Scale is then repeated. Further dose adjustments up or
down depend on the rating scale’s scores, teachers’ verbal reports, parents’
comments, and adverse effects experienced by the child. The minimal effective
total daily dose and optimal timing of medication administration should be de-
termined before switching to the sustained-release 20 mg formulation.
Eventually, one dose of standard MPH may have to be mixed with one
sustained-release 20 mg pill at 8:00 am (to give early- and late-morning
coverage), just as one administers short and long-acting insulin at the same
time.
Monitoring
Maintenance
plans should include schedules for the regular collection of information that
constitutes the child’s therapeutic drug monitoring. Each child’s response to
psychostimulants is different. Likewise, each family’s needs are different.
Plans for medication vacations and weekend and after-school dosing must be
individualized.
Medication
compliance should be monitored at each visit. The parent is instructed to bring
the medication bottle along so that pill counts can be done monthly and
compared with the prescription dates. Height and weight should be taken every 6
months, and the child’s pediatrician can be requested yearly to perform a
complete physical examination and blood work (complete blood count, liver
function studies). The frequency of visits depends on the other therapies
recommended. These may include once-monthly parental counseling, twice-monthly
individual therapy, or weekly meetings for individual psychotherapy or
behavioral modification management. A minimal frequency should be once monthly,
particularly in the nine states requiring multiple-copy prescription forms,
which limit the amount of psychostimulant ordered to a 30-day supply.
The next
component of monitoring is the use of structured
rating scales. These have been the
backbone of psychostimulant treatment
research but also have utility in clinical practice. Each physician should
choose a scale that she or he finds easy to interpret and convenient to use. It
should be available in both parent and teacher formats. These scales, however,
should not be used as substitutes for an open discussion with the teacher.
These
rating forms should be collected every 4 months and whenever the physician
needs to make decisions about dosage adjustment, time of dosing, or even
continuation of medication. Although the original 39-item Conners Teacher
Rating Scale is ubiquitous in the field of child psychopharmacology, Satin and
colleagues (1985) have shown the validity of the shorter, 10-item Abbreviated
Rating Scale both as a repeated measure and as a screening tool for identifying
school-age children with ADHD. This short form is good for tracking children in
maintenance treatment. Like most scales, the Abbreviated Rating Scale assigns
weights to each scale point. With a 10-item scale, and each point weighted
between 0 (“not at all”) and 3 (“very much”), 10 checks by a parent in the
“very much” column yields a total score of 30.
Unlike
the situation with anticonvulsants, it has not proved effective to monitor
psychostimulants by maintaining plasma concentrations within a therapeutic
range. Alternative medications such as desipramine, however, do lend themselves
to regular plasma level monitoring every 3 months.
Phases of Treatment
Medication
therapy can be divided into baseline, titration and maintenance phases. Before
the first pill is given, baseline data on height, weight, blood pressure and
heart rate should be col-lected as well as a complete blood count. The Conners
Teacher Rating Scale and Conners Parent Rating Scale can also be col-lected.
Standardized scoring methods for the teacher rating scale can be used to
generate a hyperactivity factor score.
The
duration of treatment is an important step in the generation of a treatment
plan for a child with ADHD. Although there is no clear-cut recommendation for
the length of psychostimulant treatment, many parents have a reasonable
expectation that it will not be open-ended. The onset of puberty is one
possible stopping point for psychostimulant medication. School-age children
were thought to respond “paradoxically”, calming down on stimulant medication,
an effect that was supposedly lost at puberty. Adolescents with ADHD, who may
be at risk for substance abuse, were thought to be particularly vulnerable to
psychostimulant euphoria and to abuse of their medications. In addition, more
recent prospective follow-up studies suggest that the motor hyperactivity of
ADHD disappears between ages 16 and 23 years.
Integration with Other Modalities:
Multimodal Treatment
Medication
is only one component of a comprehensive therapeu-tic treatment plan. New
standards have been promulgated for the treatment of ADHD recommending
multimodal therapy includ-ing psychological, educational and social components.
This was first reported by Satterfield and colleagues (1980) to be success-ful
in maintaining the short-term stimulant gains for periods of 2 years or more.
Related Topics