Chapter: Medical Immunology: Infections and Immunity
The Protective Role of Antibodies
THE PROTECTIVE ROLE OF ANTIBODIES
A. The Humoral Immune Response
If a pathogen is not eliminated by nonimmunological means and continues to replicate, it will eventually spread through the blood and lymph will usually be trapped by macrophages and dendritic cells in the lymph nodes and spleen. Those cells are able to internalize antigens interacting with receptors, such as the mannose receptor , pro-cess the antigen in the endosomic compartment, and express MHC-II–associated antigen-derived peptides. This creates the ideal conditions for the onset of an immune response: B lymphocytes can interact with membrane-bound antigen, while helper T cells recognize MHC-II with antigen-derived peptides presented by the same APC. The antigen-recogniz-ing cells will interact and co-stimulate each other, and after a time lag necessary for prolif-eration and differentiation of B cells into antibody-producing cells, circulating antibody will become detectable.
A primary immune response will take 5–7 days (some-times as long as 2–3 weeks) to be detected. The predominating isotype of the antibodies made early in a primary immune response is IgM, and the antibodies are of relatively low affinity. In contrast, a secondary immune response has a shorter lag phase (as short as 3–4 days), the predominant isotype of the antibodies is IgG, and the antibodies have higher affinity. These different characteristics can be exploited for diagnostic purposes. The pre-dominance of IgM or of low-affinity antibodies indicates that a given immune response has been elicited recently and that the infection is recent or ongoing.
B. Antibody-Dependent Anti-infectious Effector Mechanisms
As soon as specific antibodies become available, they can protect the organism against in-fection by several different mechanisms.
1. Complement-mediated lysis. This results from the activation of the complete se-quence of complement. However, both mammalian cells and most pathogenic microorganisms have developed mechanisms that allow them to resist complement-mediated lysis.
2. Opsonization and phagocytosis. Several proteins can opsonize and promote phagocytosis, as discussed above, but IgG antibodies are the most efficient op-sonins among the immunoglobulins. Opsonization becomes super-efficient when complement is activated as a consequence of the antigen-antibody reaction occurring on the surface of the infectious agent and C3b (the most efficient op-sonin within the complement system) joins IgG on the microbial cell membrane. This synergism is explained by the fact that Fcγ and CR1 receptors, expressed on the membranes of all phagocytic cells, mediate phagocytosis. Killing through opsonization has been demonstrated for bacteria, fungi, and viruses, while phagocytosis of antibody/complement-coated unicellular parasites has not been clearly demonstrated.
The biological significance of phagocytic cells as ultimate mediators of the effects of opsonizing antibodies is obvious; the protective effects of antibodies are lost in patients with severe neutropenia or with severe functional defects of their phagocytic cells. Those patients have increased incidence of infections with a variety of opportunistic organisms.
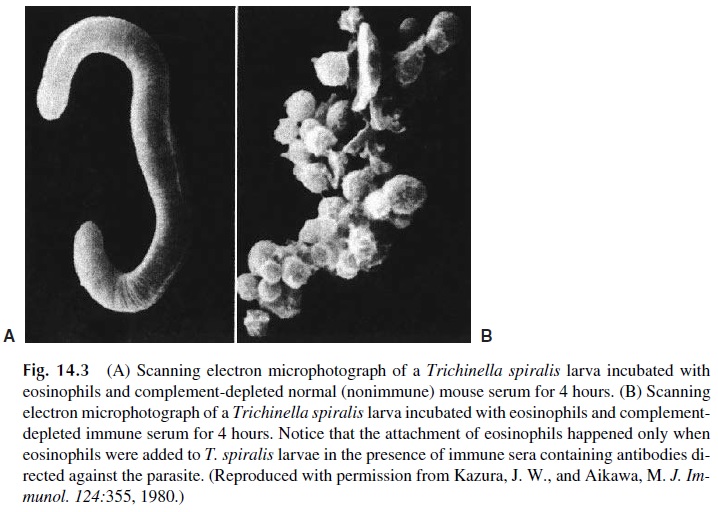
3. Antibody-dependent cell-mediated cytotoxicity (ADCC). Cells with Fc recep-tors may be able to participate in killing reactions that target antibody-coated cells. IgG1, IgG3, and IgE antibodies and cells with Fcγ R or Fce R are usually involved. Large granular lymphocytes or monocytes are the most common ef-fector cells in ADCC, but in the case of parasitic infections, eosinophils play the principal role in cytotoxic reactions (Fig. 14.3). Different effector mechanisms are responsible for killing by different types of cells. Large granular lympho-cytes kill through the release of granzymes and signaling for apoptosis; mono-cytes kill by releasing oxygen active radicals and nitric oxide; eosinophil killing is mostly mediated by the release of a “major basic protein,” which is toxic for parasites.
4. Toxin neutralization. Many bacteria release toxins, which are often the major virulence factors responsible for severe clinical symptoms. Antibodies to these toxins prevent their binding to cellular receptors and promote their elimination by phagocytosis.
5. Virus neutralization. Most viruses spread from an initial focus of infection to a target tissue via the blood stream. Antibodies binding to the circulating virus change its external configuration and prevent either its binding to cell receptors or its ability to release nucleic acid into the cell.
6. Mucosal protection. Secretory antibodies seem to play their protective role by preventing the attachment and penetration of microbial agents through mucosal surfaces.
C. Factors Influencing the Effectiveness of an Anti-infectious Humoral Response
The effectiveness of the humoral immune response in preventing an infectious disease de-pends on the time differential between the incubation period of the disease and the time needed to mount the immune response. If antibodies can be synthesized before the organ-ism proliferates or before it secretes its exotoxins, then the humoral response will prevent the infection’s clinical manifestations.
If the relevant antibodies are present in circulation as a result of vaccination, previ-ous infection, or cross-reaction between different microorganisms, protection is most ef-fective; the microorganism or its toxin(s) will be almost immediately neutralized, and the infection will remain subclinical.
If preformed antibodies are not available, protection will depend on whether antibody synthesis can take place before the “incubation period” (period of time during which the in-fectious agent is multiplying but has not yet reached sufficient mass to cause clinical dis-ease) is over. Some infectious agents, such as the influenza virus, have very short incuba-tion periods (about 2–3 days), and in such cases not even a secondary immune response can be protective. But in most infections, the duration of the incubation period is long enough to allow a secondary immune response to provide protective antibodies. Thus, for many in-fections, particularly the common viral diseases of childhood, previous exposure and ac-quisition of memory ensure that antibody will be produced in time to maintain subsequent exposures, which play the role of natural “booster” doses, explaining the “immunity for life” associated with them.
The goal of prophylactic immunization may vary from case to case. In diseases with very short incubation periods it is essential to maintain the levels of neutralizing antibody in circulation necessary to immediately abort infection. In most other diseases it may be sufficient to induce immunological memory, since once memory has been induced, the im-mune system will be able to respond in time to prevent the development of clinical infec-tion.
Finally, it must be noted that protection by humoral immunity is only possible if the infectious agent is easily available to the antibodies produced against it. Thus, intracellular pathogens are not easy to eliminate by antibodies. In addition, organisms able to change their antigenic make-up during the course of an infection can persist in spite of a vigorous humoral response.
Related Topics