Chapter: Modern Medical Toxicology: Corrosive(Caustic) Poisons: Mineral Acids (Inorganic Acids)
Sulfuric Acid - Corrosive(Caustic) Poisons

Sulfuric Acid
Synonym
Oil
of vitriol; Oleum; Battery acid.
Physical Appearance
Sulfuric acid is a heavy, oily, colourless, odourless, non-fuming liquid (Fig 5.1). It is hygroscopic, i.e. it has great affinity for water with which it reacts violently, giving off intense heat Sulfuric acid is mainly used in two forms:
·
Commercial concentrated sulfuric
acid is usually a 93–98% solution in water.
·
Fuming sulfuric acid is a solution
of sulfur trioxide in sulfuric acid.

Uses/ Sources
Sulfuric
acid is probably the most widely used industrial chem-ical in most parts of the
world including India.
·
It is used as a feedstock in the
manufacture of a number of chemicals, e.g. acetic acid, hydrochloric acid,
phosphoric acid, ammonium sulfate, barium sulfate, copper sulfate, phenol,
synthetic fertilisers, dyes, pharmaceuticals, detergents, paint, etc
·
Storage batteries utilise sulfuric
acid as an electrolyte.
·
Sulfuric acid is also used in the
leather, fur, food processing, wool, and uranium industries, for gas drying,
and as a laboratory reagent.
·
Sulfuric acid can be formed in smog
from the photo- chemical oxidation of sulfur dioxide to sulfur trioxide and
subsequent reaction with water. It is a major component of acid rain.
Usual Fatal Dose
About
20 to 30 ml of concentrate sulfuric acid. Deaths have been reported with
ingestion of as little as 3.5 ml.
Toxicokinetics
Systemic
absorption of sulfuric acid is negligible.
Mode of Action
Produces
coagulation necrosis of tissues on contact.
Clinical Features
·
Burning pain from the mouth to the stomach. Abdominal pain
is often severe.
·
Intense thirst. However, attempts at drinking water usually
provoke retching.
·
The vomitus is brownish or blackish in colour due to altered
blood (coffee grounds vomit), and may
contain shreds of the charred wall of the stomach.
·
![]() If there is coincidental damage to the larynx during
swal-lowing or due to regurgitation, there may be dysphonia, dysphagia, and
dyspnoea.
If there is coincidental damage to the larynx during
swal-lowing or due to regurgitation, there may be dysphonia, dysphagia, and
dyspnoea.
·
Tongue is usually swollen, and blackish or brownish in
colour. Teeth become chalky white. There may be constant drooling of saliva
which is indicative of oesophageal injury.
·
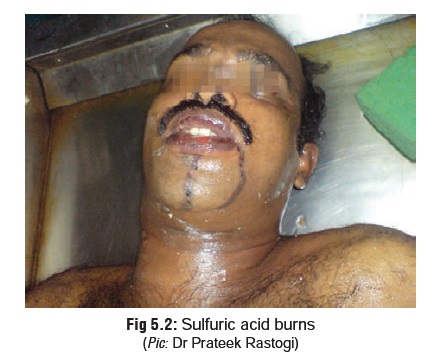
There is often acid spillage while swallowing with
conse-quent corrosion of the skin of the face (especially around the mouth),
neck, and chest (Fig 5.2). Burnt
skin appears dark brown or black.
·
Features of generalised shock are usually apparent.
·
Renal failure and decreased urine output can occur after
several hours of uncorrected circulatory collapse.
·
Because it is a strong acid, exposure to sulfuric acid may
produce metabolic acidosis, particularly following ingestion. Acidosis may be
due to severe tissue burns and shock, as well as absorption of acid.
·
Leukocytosis is common after exposure to strong mineral
acids.
·
If perforation of stomach occurs, a severe form of chemical
peritonitis can result. Rarely, perforation of duodenum (or even further down
the small intestine) may occur.
·
If the patient recovers, there are usually long-term
sequelae such as stricture formation which may lead to pyloric obstruction,
antral stenosis, or an hour glass deformity of the stomach. The oesophagus may
also be involved resulting in stenosis. There are indications of increased
propensity for carcinomas.
·
Contact with the eyes can cause severe injury including
conjunctivitis, periorbital oedema, corneal oedema and ulceration, necrotising
keratitis, and iridocyclitis.
·
Chronic
Exposure –
a.Occupational exposure to sulfuric acid mist can cause erosion of teeth over a period of time, as also increased incidence of upper respiratory infections.
b.
Sulfuric acid can react with other substances to form mutagenic and possibly
carcinogenic products such as alkyl sulfates. Case reports suggest that chronic
exposure to sulfuric acid fumes may be linked to carcinoma of the vocal cords
and nasopharyngeal carcinoma. Occupational exposure to sulfuric acid may
contribute to cases of laryngeal cancer.
Diagnosis
·
Litmus
test: The pH of the saliva can be tested with a litmuspaper to
determine whether the chemical ingested is an acid or an alkali (turns red in
acid, and blue in alkaline solution).*
·
Fresh stains in clothing may be
tested by adding a few drops of sodium carbonate. Production of effervescence
(bubbles) is indicative of an acid stain.
·
If vomitus or stomach contents are
available, add 10% barium chloride. A heavy white precipitate forms which is
insoluble on adding 1 ml nitric acid.
Treatment
·
Respiratory distress due to
laryngeal oedema should be treated with 100% oxygen and cricothyroidotomy.**
·
Some authorities recommend administration
of water or milk if the patient is seen within 30 minutes of ingestion (120–240
ml in an adult, 60–120 ml in a child). But no attempt must be made at
neutralisation with alkalis, since the resulting exothermic reaction can cause
more harm than benefit. Studies indicate that even administration of buffering
agents such as antacids can produce significant exothermic reaction.
·
Remove all contaminated clothes and
irrigate exposed skin copiously with saline. Non-adherent gauze and wrapping
may be used. Deep second degree burns may benefit from topical silver
sulfadiazine.
·
Eye injury should be dealt with by
retraction of eyelids and prolonged irrigation for at least 15 to 30 minutes
with normal saline or lactated Ringer’s solution, or tap water if nothing else
is available. Anaesthetic agents and lid retractors may be necessary. It is
desirable to continue with the irigation until normal pH of ocular secretions
is restored (7.4), which can be tested with litmus paper or urine dipstick.
Slit lamp examination is mandatory after decontamination, to assess the extent
of corneal damage.
·
The following measures are contraindicated: oral feeds, induction
of vomiting, stomach wash, and use of acti-vated charcoal.
·
Oral
feeds: Depends on degree of damage as assessedby early endoscopy.
The following is a rough guide—
a.
Mild (grade I): may have oral feedings on first day.
b.
Moderate (grade II): may have liquids after 48 to 72 hours.
c.
Severe (grade III): jejunostomy tube feedings after 48 to 72 hours.
·
Administration of steroids has been shown to delay stricture
formation (in animals) when given within 48 hours of acid ingestion, but the
practice is generally not recommended because of increased risk of perforation.
a. In case it is embarked upon, the
dosage recommended is 60 to 100 mg/day of prednisolone for the first 4 days,
followed by 40 mg/day for the next 4 days, and finally 20 mg/day for the
subsequent 7 to 10 days. In children, the appropriate dose is 2 mg/kg/day.
b.Alternatively 0.1 mg/kg of
dexamethasone or 1 to 2 mg/kg of prednisone can be given for
3 weeks and then tapered off.
·
Administer antibiotics only if
infection occurs. Prophy-lactic use is not advisable unless corticosteroid
therapy is being undertaken.
·
Since there is often severe pain,
powerful analgesics such as morphine may have to be given.
·
The use of flexible fibreoptic
endoscopy is now stan-dard practice in the first 24 to 48 hours of ingestion to
assess the extent of oesophageal and gastric damage. If there are
circumferential 2nd or 3rd degree burns, an exploratory laparotomy should be
performed. If gastric necrosis is present, an oesophagogastrectomy may have to be done.
·
Emergency laparotomy is mandatory if there is perfora-tion
or peritonitis.
·
If the patient recovers, there may be long-term sequelae
such as stenosis and stricture formation.* Follow-up is therefore essential to
look for signs of obstruction—nausea, anorexia, weight loss. Surgical
procedures such as dilatation, colonic bypass, and oesophagogastrostomy may
have to be undertaken.
Box 5.1 Management of Ocular Caustic Exposures
The most
important first-aid measure for all patients with ocular caustic exposures
should be immediate decontamination by irrigation, using copious amounts of
water or any readily available safe aqueous solution such as normal saline,
lactated Ringer’s solution, or balanced salt solution. An ocular topical
anaesthetic is usually required for effective irrigation. A complete irrigation
must be done including the conjunctival recesses, internal and external palpebral
surfaces, and corneal and bulbar conjunctiva. Lid retraction is invariably
necessary. While concern has been expressed over the use of aqueous solutions
for ocular irrigation in the case of exposure to substances which react with
water leading to heat or mechanical injury (e.g. white phosphorus), there are
hardly any documented case reports where this has actually happened.
The
duration of ocular irrigation varies with the nature of exposure. After
exposure to acids or alkalies, normalisation of the conjunctival pH is often
suggested as a useful endpoint. A minimum of 2 litres of irrigant per affected
eye should be used before any assessment of pH is done. After waiting for 5 to
10 minutes, the pH of the lower conjunctival fornix is checked. Thereafter, rechecks
are done in cycles until the pH reaches 7.5 to 8.
Adjunctival
treatment of ocular burns include application of topical antibiotic providing
antistaphylococcal and antipseudomonal coverage, cycloplegics which not only
reduce pain from ciliary spasm, but also decrease the likelihood of posterior
synechiae forma-tion, and the use of eye patches and systemic analgesics.
Topical anaesthetic agents are not desirable. Topical steroids may help lessen
the inflammation, but can interfere with healing and so must not be used for
more than the first 7 days.
In every
case of caustic ocular exposure, consultation with an ophthalmologist is
essential after administration of first-aid and decontamination, to assess
visual acuity, and to undertake slit lamp examination for detecting corneal
damage. It may take 48 to 72 hours after the burn to assess correctly the
degree of ocular damage. The basis of such an evaluation is the size of the
corneal epithelial defect, the degree of corneal opacification and extent of
limbal ischaemia.
•
Grade 1: Corneal epithelial damage; no
ischaemia.
•
Grade 2: Cornea hazy; iris details
visible, ischaemia less than one-third of limbus.
•
Grade 3: Total loss of corneal
epithelium; stromal haze obscures iris details; ischaemia of one-third to one-half
of limbus.
•
Grade 4: Cornea opaque; iris and pupil
obscured, ischaemia affects more than one-half of limbus.
Autopsy Features
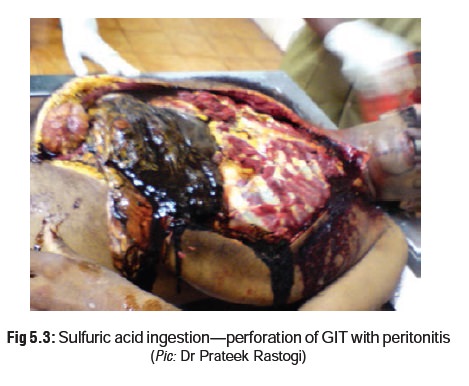
· Corroded areas of skin and mucous
membranes appear brownish or blackish. Teeth appear chalky white.
· Stomach mucosa shows the consistency
of wet blotting paper.
· There may be inflammation, necrosis,
or perforation of the GI tract (Fig 5.3).
Forensic Issues
· Accidental poisoning may arise from
mistaken identity since sulfuric acid resembles glycerine and castor oil. It is
therefore imperative that it is stored in a distinctive bottle, clearly
labelled, and kept in a safe place.
· Sulfuric acid is a rare choice for
either suicide or homicide.
· In addition to routine viscera and body
fluids, a portion of corroded skin should be cut out, placed in rectified
spirit or absolute alcohol and sent for chemical analysis. Stained clothing
must also be sent (preservative not necessary).
·
Vitriolage:
o This
term refers to the throwing of an acid on to the face or body of a person in
order to disfigure or blind him (Fig 5.4).
The motive is usually revenge or jealousy.

o Though
sulfuric acid is commonly used (hence the term vitriolage which is derived from “oil of vitriol”), other acids are
also employed. In fact any corrosive which is easy to hand may be used,
including organic acids, alkalis, and irritant plant juices.
o Going
by newspaper reports, vitriolage is a fairly common crime in India, though it
is regarded as a serious offence (grievous
hurt), and carries stiff punishment.*
Related Topics