Chapter: Modern Medical Toxicology: Corrosive(Caustic) Poisons: Mineral Acids (Inorganic Acids)
Nitric Acid - Corrosive(Caustic) Poisons

Nitric Acid
Synonym
Aqua
fortis; Azotic acid; Engraver’s acid; Hydrogen nitrate.
Physical Appearance
Nitric
acid is a colourless or yellowish fuming liquid (Fig5.5) with an acrid, penetrating odour. It is essentially
asolution of nitrogen dioxide (NO2) in water
and is available commercially in several forms.
Uses/Sources
Nitric
acid releases oxides of nitrogen into the air upon exposure to light. Therefore
exposure to nitric acid poten-tially involves exposure to oxides of nitrogen,
especially nitrogen dioxide.

Nitric
acid is formed in photochemical smog from the reaction between nitric oxide and
hydrocarbons. Individuals living in heavily polluted areas may receive chronic
inhalation exposure to nitric acid.
Workers
in the following professions may be exposed to nitrogen oxides or nitric acid:
glassblowing, engraving and electroplating, underground blasting operations,
farming (silage and fertilisers), welding, fire fighting, and industrial
chemistry.
Usual Fatal Dose
About
20 to 30 ml.
Toxicokinetics
Systemic
absorption is negligible.
Mode of Action
Nitric
acid is a powerful oxidising agent and reacts with organic matter to produce
trinitrophenol, liberating nitrogen monoxide (xanthoproteic reaction). Corrosion is less severe when compared to
sulfuric acid.
Clinical Features
The
general picture is the same as in the case of sulfuric acid, with the following
differences:
·
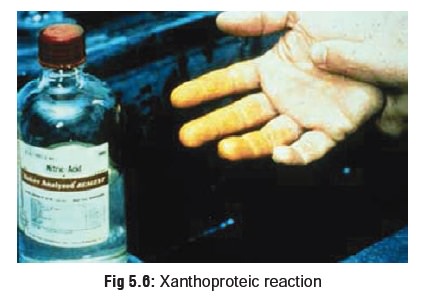
Corroded areas appear yellowish due
to xanthoproteic reaction (Fig 5.6).
Stains on clothing and teeth also appear yellowish.
·
More severe eructation and abdominal
distension due to gas formation.
·
Perforation of GI tract is less
common.
·
Inhalation of fumes can produce
coughing, rhinorrhoea, lacrimation, dyspnoea, and pulmonary oedema.
Diagnosis
·
Litmus
test.
·
Drop a small piece of copper into
the stomach contents and heat it. Pungent, dark brown heavy fumes will emanate
if nitric acid is present in sufficient concentration.
Treatment
Same
as for sulfuric acid. Respiratory distress is present more often and requires
special attention.
Autopsy Features
·
Corroded areas of skin, teeth, and
mucous membranes appear yellowish. Stains on clothing also show yellowish
discol-ouration.
·
Gastrointestinal perforation is less
common.
Forensic Issues
Same
as for sulfuric acid. Mistaking it for glycerine or castor oil, however is rare
because it is a fuming liquid.
Related Topics