Chapter: Modern Medical Toxicology: Corrosive(Caustic) Poisons: Mineral Acids (Inorganic Acids)
Hydrofluoric Acid - Corrosive(Caustic) Poisons
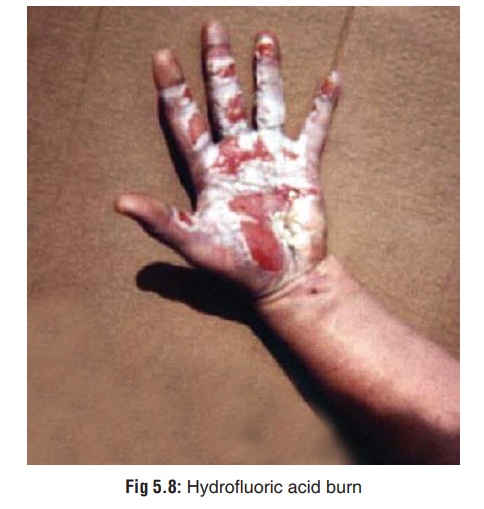
Hydrofluoric Acid
Physical Appearance
Hydrofluoric
acid is a colourless, fuming liquid. It is a unique acid, in that most of its
toxicity is due to the anion, fluoride, and not to the cation, hydrogen. Most
acids cause burns and necrosis from liberated hydrogen ions. Undiluted
hydrofluoric acid is a strong acid. Upon dilution, hydrofluoric acid is only
weakly acidic at 0.1M.
Uses
Industry:
·
90% solution: petroleum refining, pharmaceutics, and
germicides.
·
10% solution: tanning, glass and metal etching, and rust
removal.
Laboratory chemical.
Window cleaning.
Usual Fatal Dose
Unclear,
but is probably in the range of 10 to 15 ml.
Toxicokinetics
Ingestion
of hydrofluoric acid may be associated with signifi-cant systemic absorption
and manifestations such as hypocal-caemia, acidosis, and shock.
Mode of Action
Hydrofluoric
acid burns result in severe progressive tissue and bone destruction, and
excruciating pain. Unlike other inorganic acids, hydrofluoric acid rapidly
traverses the skin barrier and invades deeper tissue planes. The fluoride ion
then proceeds to affect tissue integrity and metabolism in 3 ways:
·
Liquefactive necrosis.
·
Decalcification and destruction of
bone.
·
Production of insoluble
salts—calcium and magnesium fluoride.
·
These effects result in
hypocalcaemia and hypomagnesaemia.
Clinical Features
·
Hydrofluoric acid burns can range in
severity from 1st to 3rd degree. Characteristic features
include severe pain and predilection for the sub-ungual
area (i.e. under the nail) of fingers with destruction and loss of nail, and
sometimes even the entire terminal phalanx.
1.
A hallmark of dermal exposure to low
concentrations of hydrofluoric acid is pain that is out of proportion to the
physical examination. Severe pain may be obvious, while only erythema of the
exposed skin is observed.
2.
Hydrofluoric acid readily penetrates the skin and mucous
membranes, causing deep tissue destruction (Fig 5.8). Severity and timing of effects depends on the
concentration, duration of exposure, and penetrability of the exposed tissue;
pain may be delayed.
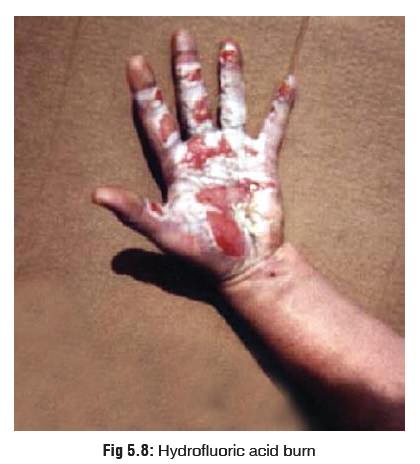
3.
The fluoride ion may cause decalcification and corro-sion of
bone beneath the area of dermal burn. Bone destruction is extremely painful.
· Inhalation causes severe throat
irritation, cough, dyspnoea, cyanosis, lung injury and noncardiogenic pulmonary
oedema.
· Ingestion is associated with severe,
burning pain followed by retching and vomiting. There is often haemorrhagic
gastritis and frank haematemesis.
· Systemic fluoride toxicity from
ingestion or dermal or injection exposure to hydrofluoric acid may result in
severe hypocalcaemia, hypomagnesaemia, hyperkalaemia, meta-bolic acidosis, and
cardiac arrhythmias (QTc prolongation, torsade de pointes, and ventricular
arrhythmias including bigeminy, ventricular tachycardia, refractory ventricular
fibrillation, and cardiac arrest). Cardiac toxicity generally manifests within
six hours of an exposure.
Treatment
1.
Patients with a history of
significant exposure or with signif-icant symptomatology should be admitted to
an intensive care unit and observed with continuous ECG monitoring for a
minimum of 24 to 48 hours.
2.
Obtain at least hourly serum
electrolytes including serial total or ionised calcium, magnesium, and
potassium levels. Total calcium may not reflect true hypocalcaemia, but usually
has a more rapid turnaround. Therapy should be directed toward signs and
symptoms of toxicity. Serum fluoride level may be used to confirm hydrofluoric
acid exposure.
3.
Obtain serial ECGs looking for signs
of hypocalcaemia (prolonged QTc interval) and hyperkalaemia (peaked T waves).
Institute continuous cardiac monitoring.
4.
Several methods have been suggested
to deactivate the injurious fluoride ion which is responsible for most of the
serious manifestations of hydrofluoric acid poisoning. The most widely accepted
method is outlined below:
First Aid:
–– Wash burnt areas copiously with water,
preferably under a shower or tap for at least 15 to 30 minutes.
––
Soak the affected portion in iced solution of 25% magnesium sulfate, or any
high molecular weight quaternary ammonium compound such as benzetho-nium or
benzalkonium.*
––
If hydrofluoric acid has been ingested, attempt immediate administration of a fluoride binding substance. Options
include milk (one-half to one glassful), chewable calcium carbonate tablets, or
milk of magnesia. Avoid large amounts of liquid, since this may induce
vomiting.
––
Stomach wash is risky and best avoided. But it may be done if
spontaneous vomiting has not occurred, and the time between ingestion and
treatment is less than 90 minutes. Addition of 10% calcium gluconate to the
lavage fluid may provide some free calcium to bind the fluoride.
–– Inhalation injury is treated by
removing the victim from the scene into fresh air, followed by decontami-nation
of the clothes and skin. The patient should be subsequently observed for signs
of laryngeal oedema, pneumonitis, and pulmonary oedema.
–– Ocular exposure should be treated
with copious irrigation of the eye for at least 30 minutes. Local ophthalmic
anaesthetic drops may be instilled to obtain patient compliance for the
prolonged irriga-tion. The pH of the eye fluid should be periodically checked
with litmus paper, and irrigation is continued until it is normal.
Subsequently, an ophthalmic consul-tation is mandatory.
Topical Skin Therapy:
––
For exposure to weak solutions of hydrofluoric acid (less than 20%),
local application of 2.5% calcium gluconate gel is the treatment of choice.
Since this gel is not available in India, it has to be prepared by the
physician by mixing 3.5 grams of calcium gluconate powder with 150 ml of a
water soluble lubricant such as K-Y jelly, which will result in a 2.5% gel.
Repeated applications may be necessary.
–– After applying the gel, an
occlusive barrier can be used (e.g. vinyl gloves or plastic wrap).
–– For skin lesions resulting from
exposure to concen-trate hydrofluoric acid, local infiltration (injection) of
10% calcium gluconate is necessary (0.5 ml/cm2 of skin, with a 30 gauge
needle).
–– Do not inject calcium chloride
into the tissues locally, since it is itself a corrosive and can accen-tuate
tissue damage. Similarly, local infiltration of magnesium sulfate or calcium
gluconate are also not recommended today by several clinicians, though there
are a few who still advocate their use. If it is decided to be done, a 10%
solution should be injected with a 30 gauge needle in amounts no greater than
0.5 ml/cm2 into and around the affected area.
Intra-arterial Therapy:
––
Hydrofluoric acid burns often occur on the fingers where intradermal calcium
injections can be hazardous. For these cases, an intra-arterial infusion
regimen has been suggested:
--
Estimate the serum calcium, magnesium, and phosphorus levels, as well as the
prothrombin time (PT), and partial thromboplastin time (PTT).
-- The appropriate artery is cannulated with a
20 gauge, 4 French or 5 French arterial catheter. If fingers are involved, the
brachial artery is cannulated; if the foot is involved, the femoral artery is
cannulated.
--
It is imperative to admit the patient to the ICU for arterial pressure wave
monitoring.![]()
-- An intra-arterial infusion of 10 ml of 10%
calcium chloride diluted with 40 ml of normal saline is given over 4 hours.
-- The
arterial wave form is checked hourly and the arterial line is flushed with
heparinised saline.
-- After infusion of the calcium chloride
solution, flush out the tubing with 10 ml of normal saline over a 15 minute
period.
-- Catheter clotting can be prevented by
adding 500 units of heparin to the infusion mixture. In case such clots do
occur, they can be lysed with 5000 units of urokinase.
-- At the end of 4 hours, the affected
extremity is checked for residual pain and tenderness. If this persists, repeat
the infusion.
-- Estimate serum calcium, magnesium,
phos-phorus, PT, PTT, 1 hour after completion of the infusion. If the magnesium
level has fallen by 0.3 mg% (or falls below 1.7 mg%), an IV infu-sion of
magnesium sulfate is begun using 1.015 mEq/hr to 4.06 mEq/hr.
-- The process of 4 hours of infusion followed
by 4 hours of rest is repeated until there is no residual tenderness to gentle
pressure.
Intravenous Therapy:
––
Regional intravenous perfusion of 5 ml of 10% calcium gluconate in 20 ml of
normal saline is reported to give immediate relief of pain in a burnt
extremity.
–– Ingestion of hydrofluoric acid
resulting in hypocal-caemia or hypomagnesaemia may require multiple IV doses of
calcium gluconate and magnesium sulfate (together with repeated cardioversion
for ventricular fibrillation) until normal blood calcium and magnesium levels
are achieved.
–– Patients should be monitored for
laboratory and/or ECG evidence of hyperkalaemia after ingestion of hydrofluoric
acid. Fluoride-induced hyperkalaemia, once developed, may be irreversible.
Therapeutic intervention to prevent development of elevated serum potassium is
essential. Quinidine has been shown to be effective in preventing the K+ efflux
from cells and preventing cardiotoxicity. Intravenous calcium has no effect on
circulating potassium levels, but it antagonises cardiac toxicity in patients
demonstrating cardiac signs and/or symptoms of hyperkalaemia. Administer
intravenous sodium bicarbonate to shift potassium intracellularly.
Ventricular arrhythmia:
Evaluate for and treat
hypocal-caemia, hypomagnesaemia and hyperkalaemia. Because amiodarone has
potassium channel blocking effects, it may be the preferred antiarrhythmic in
the setting of hydrofluoric acid poisoning.
Systemic acidosis should be
corrected with appropriate IV doses of sodium bicarbonate.Hypotension should be
managed with volume expansion and vasopressors.
Autopsy Features
Essentially
the same as for sulfuric or hydrochloric acid. There is evidence of more severe
tissue destruction.
Forensic Issues
Most
cases are due to accidental exposure at the work place.
Related Topics