Chapter: Basic & Clinical Pharmacology : Sulfonamides,Trimethoprim,& Quinolones
Sulfonamides - Antifolate Drugs
ANTIFOLATE DRUGS
SULFONAMIDES
Chemistry
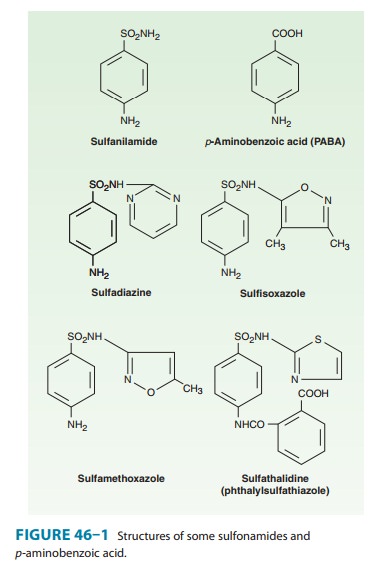
The basic formulas of
the sulfonamides and their structural similar-ity to p-aminobenzoic acid (PABA) are shown in Figure 46–1. Sulfonamides
with varying physical, chemical, pharmacologic, and antibacterial properties
are produced by attaching substituents to the amido group (–SO2–NH–R) or the amino
group (–NH2) of the sulfanilamide
nucleus. Sulfonamides tend to be much more soluble at alkaline than at acid pH.
Most can be prepared as sodium salts, which are used for intravenous
administration.
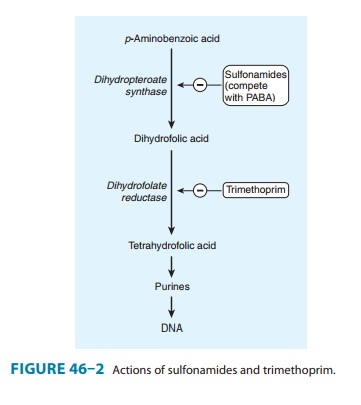
Mechanism of Action & Antimicrobial Activity
Sulfonamide-susceptible
organisms, unlike mammals, cannot use exogenous folate but must synthesize it
from PABA. This pathway (Figure 46–2) is thus essential for production of
purines and nucleic acid synthesis. As structural analogs of PABA,
sulfon-amides inhibit dihydropteroate synthase and folate production.
Sulfonamides inhibit both gram-positive and gram-negative bacteria, Nocardia sp, Chlamydia trachomatis, and some protozoa. Some enteric bacteria,
such as Escherichia coli, Klebsiella
pneumo-niae, Salmonella, Shigella,
and Enterobacter sp are also
inhibited. Itis interesting that rickettsiae are not inhibited by sulfonamides
but are instead stimulated in their growth. Activity is poor against anaerobes.
Pseudomonas aeruginosa is
intrinsically resistant to sulfonamide antibiotics.
Combination of a
sulfonamide with an inhibitor of dihydro-folate reductase (trimethoprim or pyrimethamine)
provides syn-ergistic activity because of sequential inhibition of folate
synthesis (Figure 46–2).
Resistance
Mammalian cells (and
some bacteria) lack the enzymes required for folate synthesis from PABA and
depend on exogenous sources of folate; therefore, they are not susceptible to
sulfonamides. Sulfonamide resistance may occur as a result of mutations that
cause overproduction of PABA, (2) cause production of a folic
acid-synthesizing enzyme that has low affinity for sulfonamides, or (3) impair
permeability to the sulfonamide. Dihydropteroate synthase with low sulfonamide
affinity is often encoded on a plas-mid that is transmissible and can
disseminate rapidly and widely. Sulfonamide-resistant dihydropteroate synthase
mutants also can emerge under selective pressure.
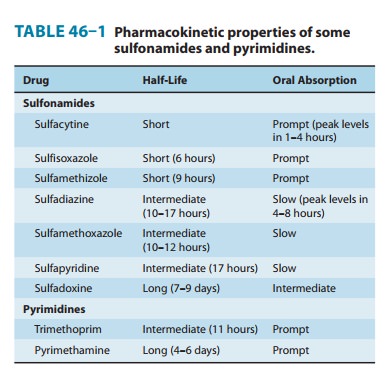
Pharmacokinetics
Sulfonamides can be
divided into three major groups: (1) oral, absorbable; (2) oral, nonabsorbable;
and (3) topical. The oral, absorbable sulfonamides can be classified as short-,
intermediate-, or long-acting on the basis of their half-lives (Table 46–1).
They are absorbed from the stomach and small intestine and distributed widely
to tissues and body fluids (including the central nervous
system and cerebrospinal fluid), placenta, and fetus. Protein bind-ing varies from 20% to over 90%. Therapeutic concentrations are in the range of 40–100 mcg/mL of blood. Blood levels generally peak 2–6 hours after oral administration.A portion of absorbed drug is acetylated or glucuronidated in the liver. Sulfonamides and inactive metabolites are then excreted into the urine, mainly by glomerular filtration. In significant renal failure, the dosage of sulfonamide must be reduced.
Clinical Uses
Sulfonamides are
infrequently used as single agents. Many strains of formerly susceptible
species, including meningococci, pneu-mococci, streptococci, staphylococci, and
gonococci, are now resistant. The fixed-drug combination of
trimethoprim-sulfamethox-azole is the drug of choice for infections such as Pneumocystisjiroveci (formerly P carinii) pneumonia, toxoplasmosis,
nocardiosis,and occasionally other bacterial infections.
A. Oral Absorbable Agents
Sulfisoxazole
and sulfamethoxazole are short- to medium-acting agents used almost exclusively
to treat urinary tract infections. The usual adult dosage is 1 g of
sulfisoxazole four times daily or 1 g of sulfamethoxazole two or three times
daily.Sulfadiazine
in combination with pyrimethamine is first-line therapy for treatment of acute
toxoplasmosis. The combination of sulfadiazine with pyrimethamine, a potent
inhibitor of dihydrofo-late reductase, is synergistic because these drugs block
sequential steps in the folate synthetic pathway blockade (Figure 46–2). The
dosage of sulfadiazine is 1 g four times daily, with pyrimethamine given as a
75-mg loading dose followed by a 25-mg once-daily dose. Folinic acid, 10 mg
orally each day, should also be adminis-tered to minimize bone marrow
suppression.
Sulfadoxine is the
only long-acting sulfonamide currently available in the USA and only as a
combination formulation with pyrimethamine (Fansidar), a second-line agent in
the treatment of malaria .
B. Oral Nonabsorbable Agents
Sulfasalazine
(salicylazosulfapyridine) is widely used in ulcerative colitis, enteritis, and
other inflammatory bowel disease .
C. Topical Agents
Sodium sulfacetamide
ophthalmic solution or ointment is effec-tive in the treatment of bacterial
conjunctivitis and as adjunctive therapy for trachoma. Another sulfonamide,
mafenide acetate, is used topically but can be absorbed from burn sites. The
drug and its primary metabolite inhibit carbonic anhydrase and can cause
metabolic acidosis, a side effect that limits its usefulness. Silver
sulfadiazine is a much less toxic topical sulfonamide and is pre-ferred to
mafenide for prevention of infection of burn wounds.
Adverse Reactions
All sulfonamides,
including antimicrobial sulfas, diuretics, diazox-ide, and the sulfonylurea
hypoglycemic agents, have been consid-ered to be partially cross-allergenic.
However, evidence for this is not extensive. The most common adverse effects
are fever, skin rashes, exfoliative dermatitis, photosensitivity, urticaria,
nausea, vomiting, diarrhea, and difficulties referable to the urinary tract .
Stevens-Johnson syndrome, although relatively uncom-mon (< 1% of treatment
courses), is a particularly serious and potentially fatal type of skin and
mucous membrane eruptionassociated with sulfonamide use. Other unwanted effects
include stomatitis, conjunctivitis, arthritis, hematopoietic disturbances ,
hepatitis, and, rarely, polyarteritis nodosa and psychosis.
A. Urinary Tract Disturbances
Sulfonamides may precipitate
in urine, especially at neutral or acid pH, producing crystalluria, hematuria,
or even obstruction. This is rarely a problem with the more soluble
sulfonamides (eg, sulfisoxazole). Sulfadiazine when given in large doses,
particularly if fluid intake is poor, can cause crystalluria. Crystalluria is
treated by administration of sodium bicarbonate to alkalinize the urine and
fluids to increase urine flow. Sulfonamides have also been implicated in
various types of nephrosis and in allergic nephritis.
B. Hematopoietic Disturbances
Sulfonamides can cause
hemolytic or aplastic anemia, granulocy-topenia, thrombocytopenia, or leukemoid
reactions. Sulfonamides may provoke hemolytic reactions in patients with
glucose-6-phosphate dehydrogenase deficiency. Sulfonamides taken near the end
of pregnancy increase the risk of kernicterus in newborns.
Related Topics