Chapter: Genetics and Molecular Biology: Xenopus 5S RNA Synthesis
Structure and Function of TFIIIA
Structure and Function of TFIIIA
One of the motivations for studying regulation of
the 5S genes was the hope that phenomena totally unheard of in prokaryotes
would be found in eukaryotes, and the first well-studied eukaryotic system
seemed likely to yield a rich harvest. For the most part, what has been found
has turned out to be phenomena that are not unique either to the 5S genes or to
eukaryotes. For example, the stable state that is determined by a protein or
complex of proteins that remain bound for long periods of time has proven to be
a valuable concept, but it is not unique either to eukaryotes or to the 5S
system. Even the startling discovery of an internal control region turns out
not to be unique, either to eukaryotes or to genes transcribed by RNA
polymerase III. The zinc fingers, which were first found in TFIIIA, are a
common structure in eukaryotes, but they also exist in prokaryotes.
The discovery of the zinc fingers is illustrative
of the unexpected source for many discoveries. While exploring the cause for
substantial losses during the purification of TFIIIA from 7S particles, workers
noticed that steps like gel filtration that could separate the protein from
small molecules led to substantial loss of TFIIIA activity. Also, addition of
metal ion chelators increased losses. Prior addition of Zn++, but not
other ions, prevented the losses. With this strong clue that zinc ion was
involved, they performed atomic adsorption spectroscopy and found indeed that
each molecule of the protein contained five to ten zinc ions.
The presence of multiple ions in the protein
suggested that an ion-binding substructure or domain might be repeated within
the pro-tein. This suspicion was reinforced by the finding that upon extended
proteolytic digestion of the protein there remained multiple species of a 3,000-4,000
dalton peptide. Upon examining the sequence of the protein for a repeated
sequence of about 30 amino acids, one was found. The marvelous property of
these repeating units was that they possessed
Figure
15.8 Locations of cysteines and
histidines in zinc fingers.
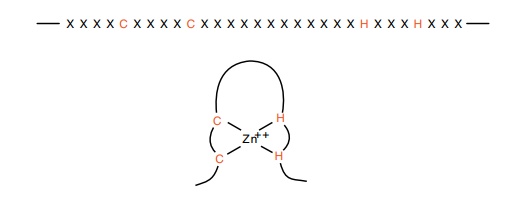
Since TFIIIA possesses nine zinc fingers, and each
can contact three bases of of the DNA, the protein can contact part of the 40
bases of the essential internal control region in the 5S gene. The portion of
the protein that does not contact DNA likely contacts one or both of the other
proteins required for most active transcription of 5S genes, TFIIIB or IIIC.
There is a 10,000 dalton portion of the protein at the N-terminal end that
probably is involved in this function since it does not possess zinc fingers
and its removal eliminates transcriptional activation by TFIIIA.
The structure of a zinc finger was predicted by
comparison with other proteins containing liganded metal ions and close
examination of the sequences of zinc fingers. Subsequent determination of the
structure of artificial zinc fingers by nuclear magnetic resonance methods and
X-ray crystallography confirmed the predicted structure.
Related Topics