Science - Solved Problems: Thermal Physics | 10th Science : Chapter 3 : Thermal Physics
Chapter: 10th Science : Chapter 3 : Thermal Physics
Solved Problems: Thermal Physics
Thermal Physics (Science)
Solved Problems
Example 1
A container whose capacity is 70 ml is filled with a liquid up to 50 ml. Then, the liquid in the container is heated. Initially, the level of the liquid falls from 50 ml to 48.5 ml. Then we heat more, the level of the liquid rises to 51.2 ml. Find the apparent and real expansion.
Data:
Level of the liquid L1 = 50 ml
Level of the liquid L2 = 48.5 ml
Level of the liquid L3 = 51.2 ml
Apparent expansion = L3 − L1
= 51.2 ml – 50 ml = 1.2ml
Real expansion = L3 − L1
= 51.2 ml – 48.5ml = 2.7ml
So, Real expansion > apparent expansion
Example 2
Keeping the temperature as constant, a gas is compressed four times of its initial pressure. The volume of gas in the container changing from 20cc (V1 cc) to V2 cc. Find the final volume V2.
Data:
Initial pressure (P1)= P
Final Pressure (P2) = 4P
Initial volume (V1) = 20cc = 20cm3 Final volume (V2) = ?
Using Boyle's Law, PV = constant P1V1 = P2V2
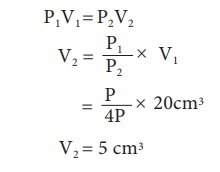
Related Topics