Chapter: 10th Science : Chapter 3 : Thermal Physics
Effect of Heat Energy
EFFECT OF HEAT ENERGY
When a certain amount of
heat energy is given to a substance, it will undergo one or more of the
following changes:
·
Temperature of the substance rises.
·
The substance may change its state from solid to liquid or from
liquid to gas.
·
The substance will expand when heated.
The rise in temperature
is in proportion to the amount of heat energy supplied. It also depends on the
nature and mass of the substance. About the rise in temperature and the change
of state, you have studied in previous classes. In the following section, we
shall discuss about the expansion of substances due to heat.
1. Expansion of Substances
When heat energy is
supplied to a body, there can be an increase in the dimension of the object.
This change in the dimension due to rise in temperature is called thermal
expansion of the object. The expansion of liquids (e.g. mercury) can be seen
when a thermometer is placed in warm water. All forms of matter (solid, liquid
and gas) undergo expansion on heating.
a) Expansion in solids
![]() When a solid is
heated, the atoms gain energy and vibrate more vigorously. This results in the
expansion of the solid. For a given change in temperature, the extent of
expansion is smaller in solids than in liquids and gases. This is due to the
rigid nature of solids.
When a solid is
heated, the atoms gain energy and vibrate more vigorously. This results in the
expansion of the solid. For a given change in temperature, the extent of
expansion is smaller in solids than in liquids and gases. This is due to the
rigid nature of solids.
The different types of
expansion of solid are listed and explained below:
1.
Linear expansion
2.
Superficial expansion
3.
Cubical expansion
1.
Linear expansion:
When a body is heated or
cooled, the length of the body changes due to change in its temperature. Then
the expansion is said to be linear or longitudinal expansion.
The ratio of increase in
length of the body per degree rise in temperature to its unit length is called
as the coefficient of linear expansion. The SI unit of Coefficient of
Linear expansion is K -1. The value of coefficient of linear
expansion is different for different materials.
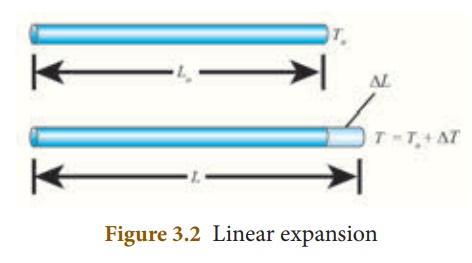
The equation relating
the change in length and the change in temperature of a body is given below:
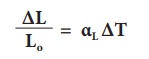
∆ L - Change in length
(Final length-Original length)
Lo - Original
length
∆T - Change in temperature
(Final temperature - Initial temperature)
αL
- Coefficient of linear expansion.
2. Superficial expansion:
If there is an increase
in the area of a solid object due to heating, then the expansion is called superficial
or areal expansion.
Superficial expansion is
determined in terms of coefficient of superficial expansion. The ratio of
increase in area of the body per degree rise in temperature to its unit area is
called as coefficient of superficial expansion. Coefficient of
superficial expansion is different for different materials. The SI unit of
Coefficient of superficial expansion is K-1
The equation relating to
the changein area and the change in temperature is given below:
∆A - Change in area
(Final area - Initial area)
Ao - Original
area
∆T - Change in
temperature (Final temperature - Initial temperature)
αA - Coefficient of superficial expansion.
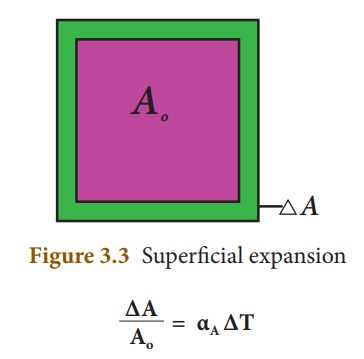
3. Cubical expansion:
If there is an increase
in the volume of a solid body due to heating, then the expansion is called cubical
or volumetric expansion.
As in the cases of
linear and areal expansion, cubical expansion is also expressed in terms of
coefficient of cubical expansion. The ratio of increase in volume of the body
per degree rise in temperature to its unit volume is called as coefficient
of cubical expansion. This is also measured in K–1.
The equation relating to
the change in volume and the change in temperature is given below:
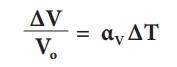
∆V - Change in
volume(Final volume - Intial volume)
Vo - Original
volume
∆T - Change in
temperature (Final temperature - Initial temperature)
αV - Coefficient
of cubical expansion.
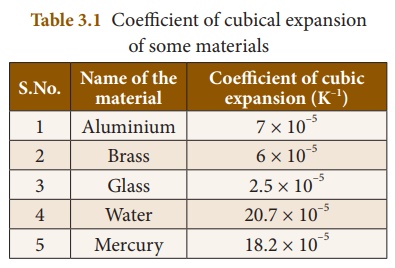
Different materials
possess different coefficient of cubical expansion. Table 3.1 gives the
coefficient of cubical expansion for some common materials.
b) Expansion in liquids and gases
When heated, the atoms
in a liquid or gas gain energy and are forced further apart. The extent of
expansion varies from substance to substance. For a given rise in temperature,
a liquid will have more expansion than a solid and a gaseous substance has the
highest expansion when compared with the other two. The coefficient of cubical
expansion of liquid is independent of temperature whereas its value for gases
depends on the temperature of gases.
When a liquid is heated,
it is done by keeping the liquid in some container and supplying heat energy to
the liquid through the container. The thermal energy supplied will be partly
used in expanding the container and partly used in expanding the liquid. Thus,
what we observe may not be the actual or real expansion of the liquid. Hence,
for liquids, we can define real expansion and apparent expansion.
1) Real expansion
If a liquid is heated
directly without using any container, then the expansion that you observe is
termed as real expansion of the liquid.
Coefficient of real
expansion is
defined as the ratio of the true rise in the volume of the liquid per degree
rise in temperature to its unit volume. The SI unit of coefficient of real
expansion is K–1.
2) Apparent expansion
Heating a liquid without
using a container is not possible. Thus, in practice, you can heat any liquid
by pouring it in a container. A part of thermal energy is used in expanding the
container and a part is used in expanding the liquid. Thus, what you observe is
not the actual or real expansion of the liquid. The expansion of a liquid
apparently observed without considering the expansion of the container is
called the apparent expansion of the liquid.
Coefficient of apparent
expansion is
defined as the ratio of the apparent rise in the volume of the liquid per
degree rise in temperature to its unit volume. The SI unit of coefficient of
apparent expansion is K–1.
2. Experiment to measure real and apparent expansion of liquid
To start with, the
liquid whose real and apparent expansion is to be determined is poured in a
container up to a level. Mark this level as L1. Now, heat the
container and the liquid using a burner as shown in the Figure 3.5.
Initially, the container
receives the thermal energy and it expands. As a result, the volume of the
liquid appears to have reduced. Mark this reduced level of liquid as L2.
On further heating, the
thermal energy supplied to the liquid through the container results in the
expansion of the liquid. Hence, the level of liquid rises to L3.
Now, the difference between the levels L1 and L3 is
called as apparent expansion, and the difference between the
levels L2 and L3 is called real expansion.
The real expansion is always more than that of apparent expansion.
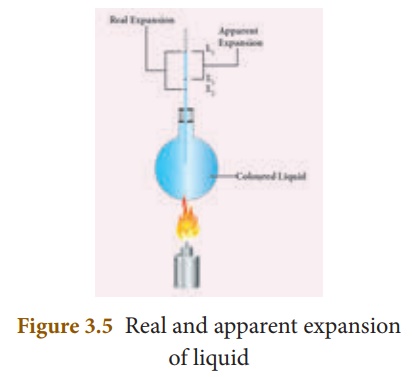
Real expansion = L3
– L2
Apparent expansion = L3
– L1
Related Topics