Chapter: Mechanical : Heat and Mass Transfer : Mass Transfer
Solved Problems: Heat and Mass Transfer - Mass Transfer
1. A vessel contains a mixture of2 kmol
of CO2 and 4.5 kmol of air at 1 bar and 25°C.ifair contains 21 % oxygen and 79%
nitrogen by volume, calculate for themixture:
(i) The
mass of CO2, O2 and N2, and the total mass;
(ii) The
percentage carbon content by mass;
(iii) The
molar mass and the gas constant for the mixture;
(iv)The
specific volume of the mixture.
Solution: (1)
Number of moles of O2 = 0.21 × 4.5 = 0.945 kmol, Number
of moles of N2 = 0.79 × 4.5 = 3.55 kmol
Mass of CO2
= 2 × 44 = 88kg; Mass of O2 = 0.945 × 32 = 30.24 kg Mass of N2
= 3.55 × 28 = 99.54 kg
The total mass = 88 + 30.24 + 99.54 = 217.48 kg
(ii) Percentage of carbon in the mixture;
(24/217.48) × 100 = 11.035% by mass.
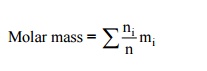
= (2/6.5)
× 44 + (0.945; 6.5) × 32 + (3.555/6.5) × 28
= 33.5
kg/kmol
And the gas constant of
the mixture; 8314/33.5 = 248.18 J/kgK (iv)Specific volume of the mixture, v =
RT/p = 248.18 × 298/ (l × 105) = 0.7395 m3/kg.
2. The air pressure inside
a synthetic rubber ball (400 mm inside diameter and 15 thick decreases from
3.5bar to 3.45bar in seven days. Estimate the coefficient of diffusion of air
in synthetic rubber if the temperature is 25°C and the solubility of air in the
rubber is 1.8 ×
10-3 kmol/m3 bar.
Solution: Since
the pressure change is very small during a period of seven days, the problem
can be treated as quasi-steady. The initial mass of air inside the ball
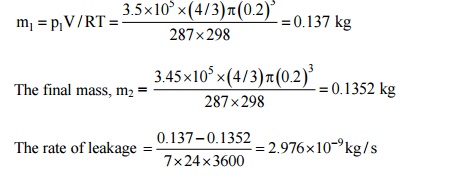
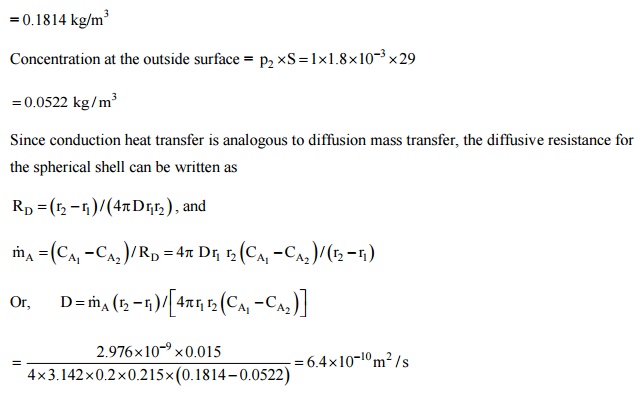
The average pressure
inside the ball = (3.45 + 3.5)/2 = 3.475 bar
Concentration inside
the ball = = 0.1814 kg/m3
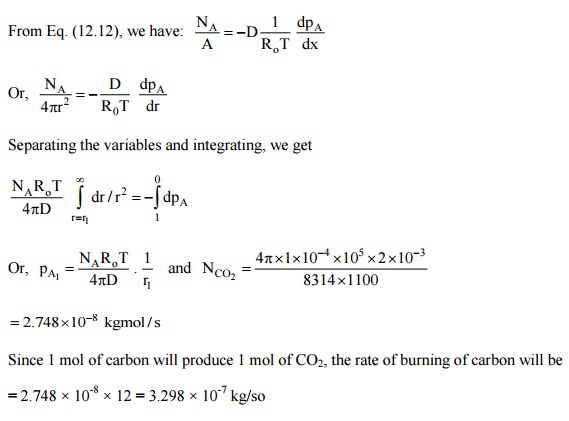
3.Estimate the rate of
burning of a pulverized carbon particle in a furnace if the diameter of the
particle is 4 mm, pressure 1 bar. The oxygen is available at 1100 K. Assume
that fairly large layer of CO2 surrounds the carbon particle. Take D
= 1 cm2/s.
Solution: The
combustion equation is C + O2®
CO2,
i.e., there will be an Equimolal counter-diffusion between O2 and CO2,
Since a fairly large blanket of carbon dioxide
surrounds the carbon particle, the partial pressure of carbon dioxide at the
surface of the carbon particle will be 1 bar and the partial pressure of oxygen
will be zero. Similarly, the partial pressure of carbon dioxide far outside
will be zero and the partial pressure of oxygen will be 1 bar.
4. Carbon dioxide at
30°C and at a pressure of 2.5bar is flowing through a rubber pipe, inside
diameter 25 mm and thickness 5 mm. The coefficient of diffusion of carbon
dioxide-rubber combination is 0.11 × 10-9 m2/s and the
solubility of carbondioxide 4 × 10-2 k mol/m3 bar.
Calculate the loss of CO2 by diffusion per unit length of the pipe.
Solution: The
species concentration at the gas-solid interface is obtained in terms of the
partial pressure of the gas adjacent to the solid surface and a
solubility factor, S.
Or, Concentration = Partial pressure × S
The carbon dioxide is
flowing through the rubber tube at 2.5bar. As such, the partial pressure of CO,
at the rubber-gas interface is 2.5bar and the concentration is then,
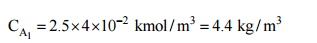
Assuming that at the
outer surface of the rubber pipe, the partial pressure of carbon dioxide is
zero, the concentration at the outer surface is zero. Further, the diffusion
mass transfer is analogous to conduction heat transfer, the diffusion
resistance in cylindrical system can be written as,
RD
=ln (r2
/ r1 )/(2 pLD)
=ln (17.5
/12.5 )/(2
´3.142 1´0.´11 10-´9)= 4.867 × 108
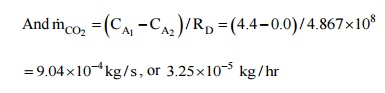
5. The air pressure inside a synthetic rubber ball (400 mm inside diameter and 15 mm thick) decreases from 3.5bar to 3.45bar in seven days. Estimate the coefficient of diffusion of air in synthetic rubber if the temperature is 25°C and the solubility of air in the rubber is 1.8 × 10-3 kmol/m3 bar. (AU2011)
Solution: Since
the pressure change is very small during a period of seven days, the problem
can be treated as quasi-steady. The initial mass of air inside the ball

Related Topics