Chapter: Mechanical : Heat and Mass Transfer : Mass Transfer
Mass Transfer
MASS TRANSFER
BASIC CONCEPTS
Air is a mixture of
various gases. Whenever we have a multicomponent system with a concentration
gradient, one constituent of the mixture gets transported from the region of
higher concentration to the region of lower concentration till the
concentration gradient reduces to zero. This phenomenon of the transport of
mass as a result of concentration gradient is called 'Mass Transfer'.
The mass transfer
phenomenon is analogous to heat transfer phenomenon. In heat transfer - heat
energy flows in a direction of decreasing temperature gradient and ceases when
the temperature gradient reduces to zero. In mass transfer - the transfer of
mass takes place in the direction of decreasing concentration gradient and
ceases when the concentration gradient is zero.
The.
common examples of mass transfer in our everyday life and in many
industries
are:
- diffusion
of smoke discharged by tall chimney into the atmosphere,
- a
drop of ink diffusing in a glass of still water,
- evaporation
of a drop of perfume in a room,
- humidification
of air flowing over a spray pond or cooling tower,
- mixing
of diesel or petrol with air inside an internal combustion engine,
- diffusion
welding of metals,
- diffusion
of neutron in a nuclear reactor.
Different Modes of Mass
Transfer
There
are basically two modes of mass transfer:
(i)
Mass Transfer by Diffusion - the
transport of mass by random molecular motion in quiescent or laminar flowing
fluids is known as mass transfer by 'diffusion' and is analogous to heat
transfer by conduction. Mass transfer by diffusion occurs due to (a) concentration
gradient,
(b) temperature
gradient, and (c) hydrostatic pressure difference.
(ii)
Convective Mass Transfer - the rate of
molecular diffusion of mass can be accelerated by the bulk motion of the fluid.
Mass can be transported between the boundary of a surface and a moving fluid
(drying of clothes, molecular diffusion of a sugar cube in a cup of coffee by
stirring, moist air flowing over the surface of an ocean and precipitation on a
dry land etc.), or between two moving fluids which are relatively immiscible
(formation of clouds, vapourisation of water in a tea kettle). This mechanism
of mass transfer is called 'convectIve mass transfer' and is analogous to heat
transfer by convection (free or forced).
Dalton's Law of Partial
Pressure
Each constituent of a multicomponent system
contributes to the total pressure by an amount which is known as the 'partial
pressure' of the constituent. The relationship between the partial pressures of
the constituents is expressed by Dalton's Law:
The pressure of a
mixture of gases is equal to the sum of the partial pressure of the
constituents. The partial pressure of each constituent is that pressure which
the gas would exert if it occupied alone that volume occupied by the mixture at
the same temperature.
For a mixture of ideal
gases, we have
P = PA + PB
+ ...... + PK;
where PA is
the partial pressure of the species A and so on.
= PD ……….(5.1)
Dalton's law was
reformulated by Gibbs to include a second statement on the properties of
mixtures. The combined statement is Gibbs-Dalton law:
The internal energy.
enthalpy and entropy of a gaseous mixture are respectively equal to the sum of
the internal energies, enthalpies, and entropies of the constituents. The
internal energy, enthalpy and entropy which a constituent would have if it
occupied alone that volume occupied by the mixture at the temperature of the
mixture.
Molar Density, Mass
Density, Mass Fraction and Mole Fraction
There are a number of
ways by which the concentration for a species in a multicomponent mixture can
be defined:
(i) Molar Density or
Molar Concentration, CA = number of moles of the species A per unit
volume of mixture, kg-mol/m3
A = mass of the species
A per unit volume of the mixture, kg/m3.
(iii)
Mass Fraction, mA = mass concentration of component A / total mass
density of the
mixture.
(iv) Mole Fraction, XA
= number of moles of species A / total number of moles of the mixture. = CA/C
Therefore,
the following summation rules hold true:
CA + CB
+ .... + CK = C
CA + CB + .... + CK = C
XA + XB
+ ..... + XK = I1
rnA + mB
+ ..... + mK = I (5.2)
Since the number of
moles = mass of species/molecular weight, we have
C AA = r/
MA
For
a perfect gas, we have:
PA
V =n A R oT ,
where Ro is the universal gas constant,
and, CA =n
A / V =PA / R oT
X
A =CA / C =PA
/ P and C =p / R oT
Mass Average and Molar Average Velocities and
Different Types of Fluxes Velocity
In a multicomponent
mixture, the bulk velocity of the mixture can be defined on the basis of mass
average or molar average velocity. Let VA A
is the mass density of the species A, then the mass average velocity would be:
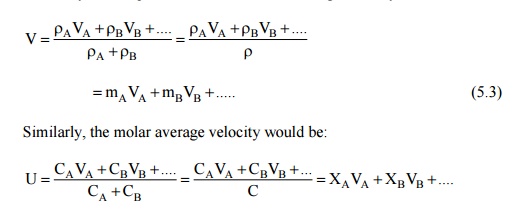
Since mass transfer
requires the diffusion of a species with respect to a plane moving with an
average velocity, diffusion will take place when the diffusion velocity is in
excess of the average velocity. Thus
Mass diffusion velocity of the species A : VA –V (5.4)
Molar diffusion velocity of the species A : VA - U (5.5)
Fluxes: The mass flux
of species A can be expressed relative to either a fixed observer or an
observer moving with the bulk velocity. For a stationary observer, the absolute
flux of any species A will be equal to the sum of the flux due to the molecular
diffusion and that due to the bulk motion.

Similarly,
molar diffusion flux = CA(VA –V)
Fick's Law of Diffusion
The fundamental
equation (one-dimensional) of molecular diffusion is known as Fick's law. It
has been derived from the kinetic theory of gases, and can be written for a
binary mixture as
JA
= –DAB (d CA/dx) (5.8)
where DAB =
diffusion coefficient of
species A with
respect to species
B,
JA = molar flux in the X-direction
relative to the molar average velocity,
dCA/dx
= Concentration gradient in X-direction.
Let us consider a two
compartment tank as shown in Fig. 5.1. One compartment contains gas A and the
other compartment contains gas B and both the compartments are initially at a
uniform pressure and temperature throughout. When the partition between the
compartments is removed, the two gases will diffuse through each other until
equilibrium is established and the concentration of the gases is uniform
throughout the tank.
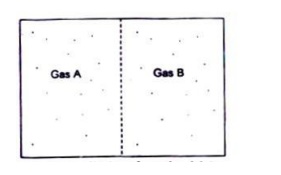
Fig.
5.1 Diffusion of species A in to species B
Fig 5.2 illustrates the
dependence of diffusion on the concentration profile. The concentration of the
species A on the left side of the imaginary plane is greater than that on the
right side. As such, more molecules will cross the plane per unit time from
left to right. This would lead to a net transfer of mass from the region of
higher concentration to the region of lower concentration.
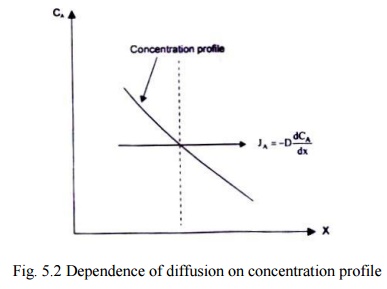
Fig.
5.2 Dependence of diffusion on concentration profile
* This law assumes that
fluxes are measured relative to the coordinates that move with the average
velocity of the mixture.
CONVECTIVE MASS TRANSFER :
(i) Diffusion in Gases
- the diffusion rates in gases are dependent on the molecular speed which is a
function of temperature and therefore, the diffusion coefficient depends upon
the temperature of gases.
Gilliland has proposed
a semi-empirical equation for diffusion coefficient in a binary gas mixture –
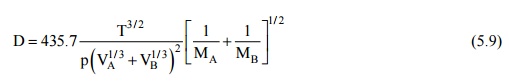
where D is in square
centimeters per second, T is in Kelvin, p is the total pressure of the system
in pascals, VA and VB are the molecular volumes of the
species A and R as calculated from the atomic volumes in Table 12.1, MA
and MB are the molecular weights of species A and B.
Diffusion coefficients
for gases depend upon pressure, temperature and other molecular properties of
diffusing gases. At two different pressure and temperature, we have
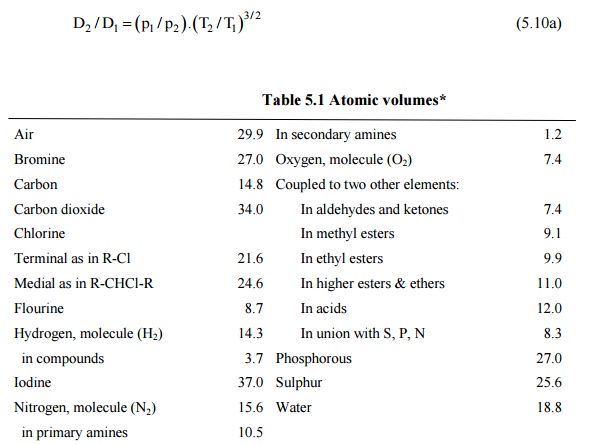
Table 5.1 Atomic
volumes*
Air 29.9 In
secondary amines 1.2
Bromine 27.0 Oxygen,
molecule (O2) 7.4
Carbon 14.8 Coupled
to two other elements:
Carbon dioxide 34.0 In
aldehydes and ketones 7.4
Chlorine In methyl esters 9.1
Terminal as in R-Cl 21.6 In
ethyl esters 9.9
Medial as in R-CHCl-R 24.6 In
higher esters & ethers 11.0
Flourine 8.7 In
acids 12.0
Hydrogen, molecule (H2) 14.3 In
union with S, P, N 8.3
in compounds 3.7 Phosphorous 27.0
Iodine 37.0 Sulphur 25.6
Nitrogen, molecule (N2) 15.6 Water 18.8
in primary amines 10.5
*(For three numbered
ring like ethylene oxide, deduct 6.0, for four numbered ring like cyclobutane,
deduct 8.5, for six numbered ring like benzene, deduct 15.6, for napthelene
ring, deduct 30.0.)
(ii) Diffusion in
Liquids and Solids - Diffusion in liquids occurs at much slower rate than in
gases. Since kinetic theory of liquids is not as much developed as that of
gases, it is usually assumed as a first approximation that equations of the
same general form are applicable to the diffusion of a solute in a solvel1t as
to the diffusion in gases, i.e., Fick's law is assumed valid for liquids.
Diffusion coefficient
for most of the common organic and inorganic materials in the
usual solvents such as
water, alcohol and benzene at room temperature lie m the range of 1.79 × 10-3
to 1.075 × 10-7 cm2/s.
Diffusion in solids is
much slower than in liquids. Diffusion of solids in solid has limited
engineering applications but diffusion of fluids in solids have extensive
applications. Fick's law is sometimes used, with an empirically determined
effective diffusivity which takes care of the structure of solid. A typical
problem of liquid transfer in a solid, of interest, is drying of solids.
HEAT AND MASS TRANSFER ANALOGY:
Fick's law (Eq. 5.8)
can also be expressed in terms of mass flux per unit area or mass concentration
or in terms of molal concentrations and fluxes. For gases, the law may be
expressed in terms of partial pressures by making use of the perfect gas
equation of state:
Since the
characteristic gas constant of a gas is: RA = Ro/MA
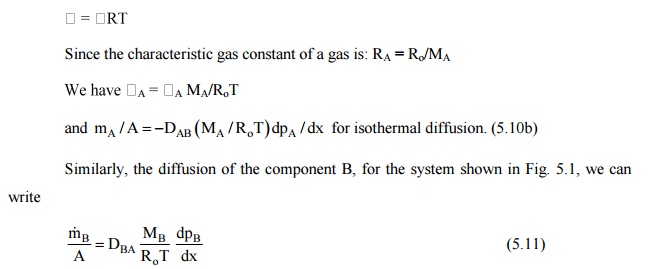
When we have equimolal
counter diffusion, shown in Fig. 12.3 (a, b), the steady state molal diffusion
rates of the species A and B, represented by NA and NB
will be given by
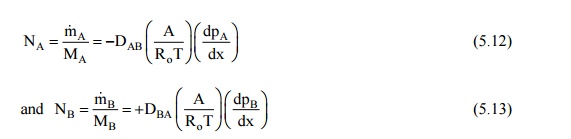
The total pressure of
the system remains constant at steady state,
or, p = pA +
pB ; and dpA/dx +
dpB/dx = 0
as dpA/dx = –dpB/dx
Since each molecule of
A is replaced by a molecule of B, the molal diffusion rates must be equal.
Thus: NA = –NB, and
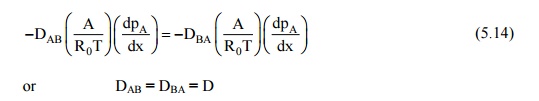
Or
DAB = DBA = D
This fact is known as
the equivalence of diffusion coefficients or diffusivities in binary mixtures,
and is a property of the binary mixture.
By integrating Eq.
(12.10), we can obtain the mass flux of the species A as;

corresponding to the
nomenclature used in Fig. 5.3 (a, b). Table 5.2 gives the values of the binary
diffusion coefficients.
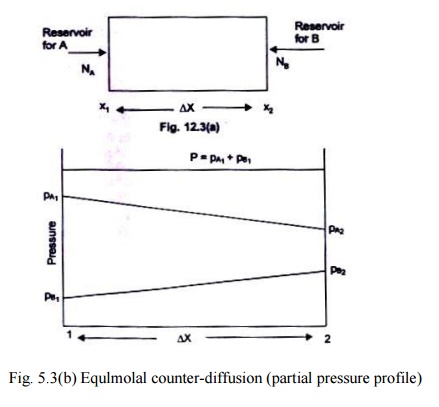
Fig. 5.3(b) Equlmolal
counter-diffusion (partial pressure profile)
(iv) It does not tell
about diffusion due to temperature or pressure gradient or due to external
forces.
CONVECTIVE MASS TRANSFER CORRELATIONS :
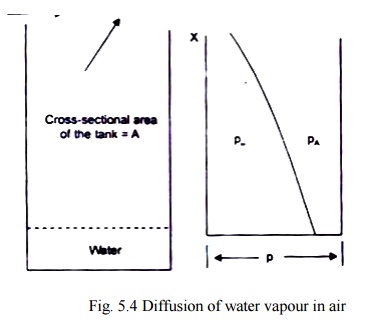
Let us consider a tank
containing water which is exposed to air in the tank as shown in Fig. 5.4. We
assume that:
(i) the
system is isothermal,
(ii) the
total pressure remains constant,
(iii)
the system is in steady state. Since
there has to be a little movement of air over the top of the tank to remove the
water vapour that diffuses to that point, the air movement does not create any
turbulence to alter the concentration profile in the tank, and
air and water vapour
both behave like ideal gases.
From Eq. (5.10), the
downward diffusion of air can be written as
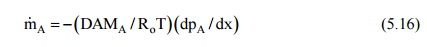
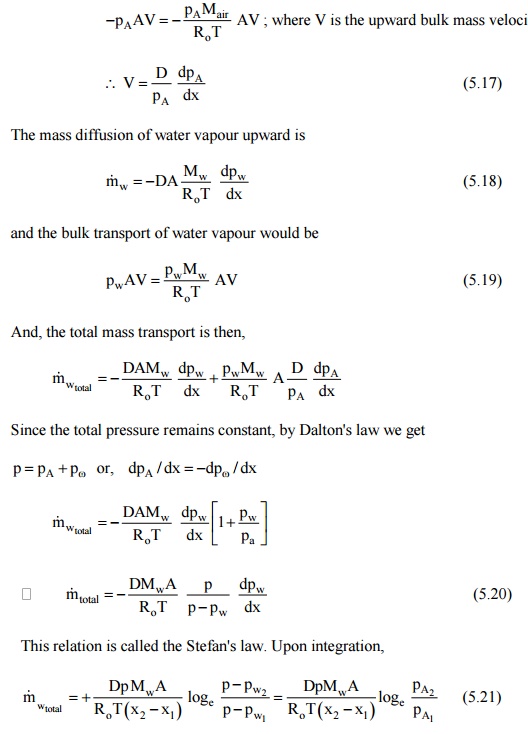
and this has to be
balanced by the bulk mass transfer upward. Therefore,
Related Topics