Chapter: Essentials of Psychiatry: Schizophrenia and Other Psychoses
Schizophrenia: Psychopharmacological Treatment
Psychopharmacological Treatment
Background
The seminal work of Delay and Deniker (1952) provided a phar-macological
strategy that would forever change the face of schiz-ophrenia. The
implementation of chlorpromazine became the turning point for
psychopharmacology. Patients who had been institutionalized for years were able
to receive treatment as out-patients and live in community settings. The road
was paved for the deinstitutionalization movement, and scientific
understand-ing of the pathophysiology of schizophrenia burgeoned. The
in-troduction of clozapine started the era of antipsychotic agents be-ing
referred to as either “typical” (conventional
or traditional) or “atypical” (or novel) antipsychotic drugs. If
chlorpromazine started the first revolution in the psychopharmacological
treat-ment of schizophrenia, then clozapine ushered in the second and more
profound revolution whose impact is felt beyond schizo-phrenia and its full
extent is yet to be realized. Moreover, cloza-pine has invigorated the
psychopharmacology of schizophrenia and rekindled one of the most ambitious
searches for new antip-sychotic compounds by the pharmaceutical industry. There
are now five novel antipsychotics in the USA: risperidone, olanzap-ine,
quetiapine, ziprasidone and aripiprazole.
Clozapine and the Novel Antipsychotic Agents
Double–blind, controlled studies demonstrated the superior clin-ical
efficacy of clozapine compared with standard neuroleptics, without the
associated extrapyramidal symptoms. It is clearly superior to traditional
neuroleptics for psychosis. Approximately 50% of patients with chronic and
treatment-resistant schizophre-nia derive a better response from clozapine than
from traditional neuroleptics. Its effect on negative symptoms is somewhat
con-troversial and has started an intense and a passionate debate as to whether
the efficacy of the medication is with primary or secondary negative symptoms
or both (Meltzer, 1989a). There is substantial evidence that clozapine
decreases relapses, improves stability in the community, and diminishes
suicidal behavior. There have also been reports that clozapine may cause a
gradual reduction in preexisting tardive dyskinesia.
Unfortunately,
clozapine is associated with agranulocyto-sis and, because of this risk, it
requires weekly white blood cell testing. Approximately 0.8% of patients taking
clozapine and re-ceiving weekly white blood cell monitoring develop
agranulocy-tosis. Women and the elderly are at higher risk than other groups.
The period of highest risk is the first 6 months of treatment. These data has
led to monitoring of white cell counts less frequently after first 6 months to
every other week if a person has a history of white cell counts the within the
normal range in the preceding 6 months. Current guidelines state that the
medication must be held back if the total white blood cell count is 3000/mm3 or less
or if the absolute polymorphonuclear cell count is 1500/mm3 or less.
Patients who stop clozapine treatment continue to require bloodmonitoring for at least 4 weeks after the last dose according to current
guidelines. Other side effects of clozapine include orthos-tatic hypotension,
tachycardia, sialorrhea, sedation, elevated tem-perature and weight gain.
Furthermore, clozapine can lower the seizure threshold in a dose-dependent
fashion, with a higher risk of seizures seen particularly at doses greater than
600 mg/day.
Clozapine has an affinity for dopamine receptors (D1, D2, D3, D4 and D5), serotonin receptors (5-HT2A, 5-HT 2C, 5-HT6 and 5-HT7), alpha-1- and alpha-2-adrenergic receptors, nicotinic and muscarinic cholinergic receptors and H1 histaminergic receptors. As
clozapine has a relatively shorter half-life, it is usually admin-istered twice
a day.
The superior antipsychotic efficacy of clozapine has in-spired an
abundance of research in the field of modern psychop-harmacology for the
treatment of schizophrenia. Clozapine and the other novel compounds have an
array of biochemical profiles, with affinities to dopaminergic, serotoninergic
and noradrener-gic receptors. Research on the atypical antipsychotic compounds
has led to a greater understanding of the biochemical effects of antipsychotic
agents, leaving the basic dopamine hypothesis of schizophrenia insufficient to
explain schizophrenic symptoms. Clozapine shows selectivity for mesolimbic
neurons and does not increase the prolactin level. Binding studies have shown
it to be a relatively weak D1 and D2 antagonist, compared with traditional neuroleptics (Farde et al., 1989). Clozapine shares the
property of higher serotonin 5-HT2A to dopamine D2 blockade ratio reported to impart atypicality. The noradrenergic system
may also have a role in the mechanism of action of clozapine (Breier, 1994).
Clozapine, but not traditional neuroleptics, causes up to fivefold increases in
plasma norepinephrine. Moreover, these increases in norepinephrine correlated
with clinical response.
Risperidone
is a benzisoxazol compound with a high affin-ity for 5-HT2A and D2
receptors and has a high serotonin dopamine receptor antagonism ratio. It has
high affinity for alpha-1-adren-ergic and H1
histaminergic receptors and moderate affinity for alpha-2-adrenergic receptors.
Risperidone is devoid of significant activity against the cholinergic system
and the D1
receptors. The efficacy of this medication is equal to that of other first-line
atypi-cal antipsychotic agents and is well tolerated and can be given once or
twice a day. It is available in a liquid form as well. The most common side
effects reported are drowsiness, orthostatic hypotension, lightheadedness, anxiety,
akathisia, constipation, nausea, nasal congestion, prolactin elevation and
weight gain. At doses above 6 mg/day EPS can become a significant issue. The
risk of tardive dyskinesia at the regular therapeutic doses is low.
Olanzapine, a thienobenzodiazepine compound, has an-tagonistic effects
at dopamine D1 through D5 receptors and sero-tonin 5-HT2A, 5-HT2C and 5-HT6 receptors. The antiserotonergic activity is more potent than the
antidopaminergic one. It also has affinity for alpha-1-adrenergic, M1 muscarinic acetylcholinergic and
H1 histaminergic receptors. It differs from clozapine by not having high
affinity for the 5-HT7, alpha-2-adrenergic and other cholinergic receptors. It has significant
efficacy against positive and negative symptoms and also improves cognitive
functions. EPS is minimal when used in the therapeutic range with the
ex-ception of mild akathisia. As the compound has a long half-life, it is used
once a day and as it is well tolerated, it can be started at a higher dose or
rapidly titrated to the most effective dose. It is available as a rapidly
disintegrating wafer form, which dis-solves immediately in the mouth, and as an
intramuscular form. The major side effects of olanzapine include significant
weight gain, sedation, dry mouth, nausea, lightheadedness, orthostatichypotension,
dizziness, constipation, headache, akathisia and transient elevation of hepatic
transaminases. The risk of tardive dyskinesia and NMS is low. Though used as a
once-a-day medi-cation, it is often administered twice a day with the average
dose of 15 to 20 mg/day. However, doses higher than 20 mg/day are of-ten used
clinically and are thus being evaluated in clinical trials.
Quetiapine,
a dibenzothiazepine compound has a greater affinity for serotonin 5-HT2 receptors than for dopamine D2
recep-tors; it has considerable activity at dopamine D1, D5, D3, D4,
se-rotonin 5-HT1A, and
alpha-1-, and alpha-2-adrenergic receptors. Unlike clozapine, it lacks affinity
for the muscarinic cholinergic receptors. It is usually administered twice a
day due to a short half-life. Quetiapine is as effective as typical agents and
also appears to improve cognitive function. Among 2035 patients enrolled in
seven controlled studies, quetiapine at all doses used did not have an EPS rate
greater than placebo. This is in contrast to olanzapine, risperi-done and
ziprasidone, where there were dose related effects on EPS levels. The rate of
treatment emergent EPS was very low even in high at-risk populations such as
adolescent, parkinsonian patients with psychosis and geriatric patients. Major
side effects include somnolence, postural hypotension, dizziness, agitation,
dry mouth and weight gain. Akathisia occurs on rare occasions. The package
insert warns about developing lenticular opacity or cataracts and advises
periodic eye examination based on data from animal stud-ies. However, recent
data suggest that this risk may be minimal.
Ziprasidone
has the strongest 5-HT2A receptor
binding rela-tive to D2 binding
amongst the atypical agents currently in use. Interestingly, ziprasidone has
5-HT1A agonist
and 5-HT1D
antago-nist properties with a high affinity for 5-HT1A, 5-HT2C and 5-HT1D receptors. As it does not interact with many other
neurotransmit-ter systems, it does not cause anticholinergic side effects and
pro-duces little orthostatic hypotension and relatively little sedation. Just
like some antidepressants, ziprasidone blocks presynaptic reuptake of serotonin
and norepinephrine. Ziprasidone has a rela-tively short half-life and thus it
should be administered twice a day and along with food for best absorption.
Ziprasidone is not completely dependent on CYP3A4 system for metabolism, thus
inhibitors of the cytochrome system do not significantly change the blood
levels. Ziprasidone at doses between 80 and 160 mg/ day is probably the most
effective agent for treating symptoms of schizophrenia. To assess the cardiac
risk of ziprasidone and other antipsychotic agents a landmark study was
designed to evaluate the cardiac safety of the antipsychotic agents, given at
high doses alone and with a known metabolic inhibitor in a randomized study
involving patients with schizophrenia. This was done to replicate the possible
worst-case scenario (overdose or dangerous combina-tion treatment) in the real
world. All antipsychotic agents studied caused some degree of QTc prolongation,
with the oral form of ha-loperidol associated with the least and thioridazine
with the great-est degree. Major side effects reported with the use of
ziprasidone are somnolence, nausea, insomnia, dyspepsia and prolongation of QTc
interval. Dizziness, weakness, nasal discharge, orthostatic hypotension and
tachycardia occur less commonly.
Ziprasidone should not be used in combination with other drugs that
cause significant prolongation of
the QTc interval. It is also contraindicated for patients with a known history
of signifi-cant QTc prolongation, recent myocardial infarction, or symp-tomatic
heart failure. Ziprasidone has low EPS potential, does not elevate prolactin
levels and causes approximately 1 lb weight gain in short-term studies.
At present, with respect to efficacy, it does not appear that any one of
the novel antipsychotic agents (except clozapine) isbetter
than another one in treating schizophrenia. The randomized controlled trials
suggest that, on average, these antipsychotic agents are each associated with
20% improvement in symptoms. However, clozapine is the only new antipsychotic
agent that is more effective than haloperidol in managing treatment resistant
schizophrenia. Unfortunately, its potential for treatment-emergent
agranulocytosis, seizures and the new warning of myocarditis, precludes its use
as a first line agent for schizophrenia. A major difference amongst the newer antipsychotic
agents is the side effect profile and its effect on the overall quality of life
of the patient.
Acute Treatment
In last few years, the use of novel antipsychotics has surpassed the use
of typical ones in the management of acute phase symptoms of schizophrenia,
except for the use of parenteral and liquid forms of antipsychotics where
typical antipsychotic agents still hold an upper hand. However, this trend will
most likely change once the injectable preparations of the novel antipsychotics
enter the market. The primary goal of acute treatment is the ameliora-tion of
any behavioral disturbances that would put the patient or others at risk of
harm. Acute symptom presentation or relapses are heralded by the recurrence of
positive symptoms, including delusions, hallucinations, disorganized speech or
behavior, se-vere negative symptoms or catatonia. Quite frequently, a relapse
is a result of antipsychotic discontinuation, and resumption of an-tipsychotic
treatment aids in the resolution of symptoms. There is a high degree of
variability in response rates among individuals. When treatment is initiated,
improvement in clinical symptoms can be seen over hours, days, or weeks of
treatment.
Studies have shown that although typical neuroleptics are undoubtedly
effective, a significant percentage (between 20 and 40%) of patients show only
a poor or partial response to tradi-tional agents. Furthermore, there is no
convincing evidence that one typical antipsychotic is more efficacious as an
antipsychotic than any other, although a given individual may respond better to
a specific drug. Once an informed choice has been made between using a novel or
typical antipsychotic medication by the patient and the clinician, selection of
a specific antipsychotic agent should be based on efficacy, side-effect
profile, history of prior response (or nonresponse) to a specific agent, or
history of response of a family member to a certain antipsychotic agent. (For a
pharmacotherapy decision tree based on Texas Medication Algorithm Project, see
Figure 45.3.) Amongst the typical antipsychotic medications, low-potency, more
sedating agents, such as chlorpromazine, were long thought to be more effective
for agitated patients, yet there are no consistent data proving that high-potency
agents are not equally useful in this context. The low-potency antipsychotics,
however, are more associated with orthostatic hypotension and lowered sei-zure
threshold and are often not as well tolerated at higher doses. Higher potency
neuroleptics, such as haloperidol and fluphena-zine, are safely used at higher
doses and are effective in reducing psychotic agitation and psychosis itself.
However, they are more likely to cause EPS than the low potency agents.
The
efficacy of novel antipsychotic drugs on positive and negative symptoms is
comparable to or even better than the typi-cal antipsychotic. The significantly
low potential to cause EPS or dystonic reaction and thus the decreased
long-term consequences of TD has made the novel agents more tolerable and
acceptable in acute treatment of schizophrenia. Other significant advantages
adding to the popularity of novel antipsychotics include their ben-eficial
impact on mood symptoms, suicidal risk and cognition. The selection of the
first line treatment with novel antipsychotic
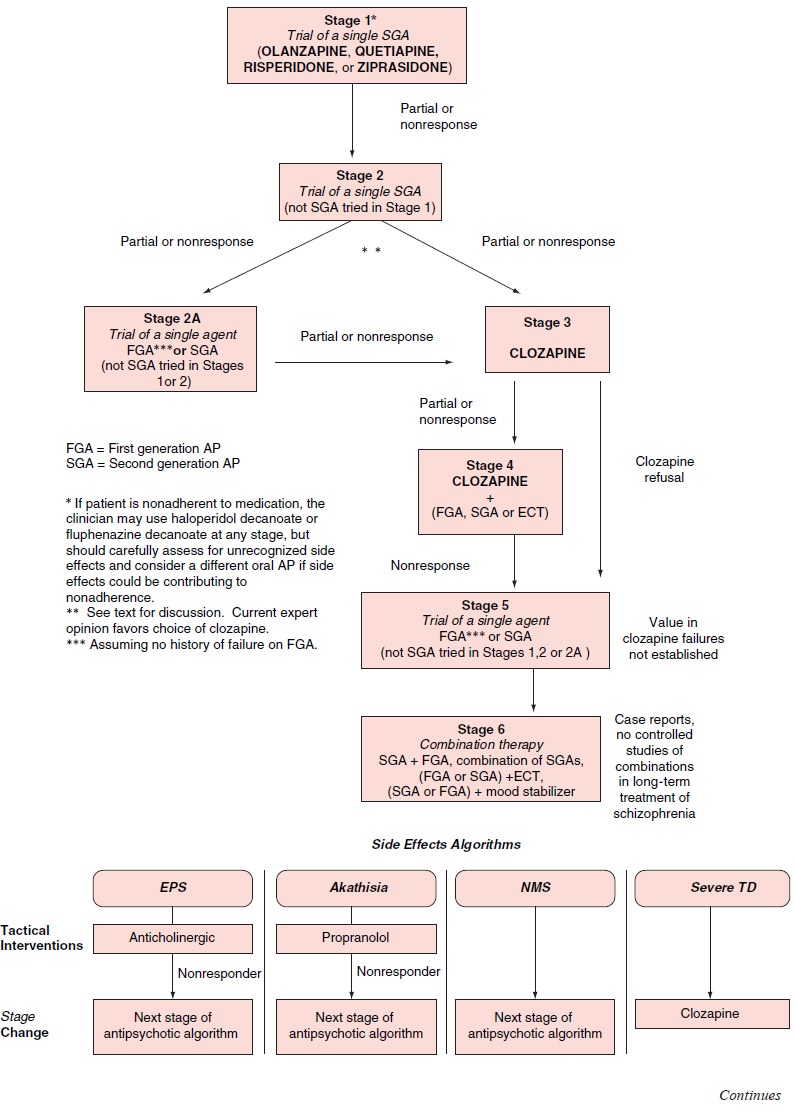
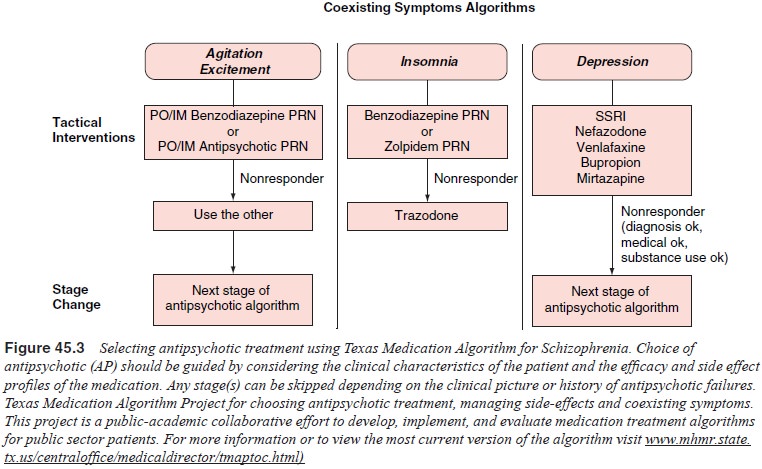
(and occasionally typical antipsychotic agent) also depends on the
circumstances under which the medications are started, for example, extremely
agitated or catatonic patients would require intramuscular preparation of the
antipsychotic agents which would limit the choice. Except for clozapine, which
is not con-sidered first line treatment because of substantial and potentially
life threatening side effects, there is no convincing data support-ing the
preference of one atypical over the other. However, if the patient does not
respond to one, a trial with another atypical an-tipsychotic is reasonable and
may produce response.
Once the decision is made to use an antipsychotic agent, an appropriate
dose must be selected. Initially, higher doses or re-peated dosing may be
helpful in preventing grossly psychotic and agitated patients from doing harm.
In general, there is no clear evidence that higher doses of neuroleptics (more
than 2000 mg chlorpromazine equivalents per day) have any advantage over
standard doses (400–600 chlorpromazine equivalents per day).
Some patients who are extremely agitated or aggressive may benefit from
concomitant administration of high-potency benzodiazepines such as lorazepam,
at 1 to 2 mg, until they are stable. Benzodiazepines rapidly decrease anxiety,
calm the person, and help with sedation to break the cycle of agitation. They
also help decrease agitation due to akathisia. The use of these medications
should be limited to the acute stages of the ill-ness to prevent tachyphylaxis
and dependency. Benzodiazepines are quite beneficial in treatment of catatonic
or mute patients but the results are only temporary though of enough duration
to help with body functions and nutrition.
Maintenance Treatment
There is by now a great deal of
evidence from long-term follow-up studies that patients have a higher risk of
relapse and exacerbations if not maintained with adequate antipsychotic
regimens. Noncom-pliance with medication, possibly because of intolerable
neu-roleptic side effects, may contribute to increased relapse rates.
Indouble-blind, placebo-controlled study of relapse rates, 50% of patients in a
research ward demonstrated clinically significant ex-acerbation of their
symptoms within 3 weeks of stopping neurolep-tic treatment. It is estimated
that two-thirds of patients relapse af-ter 9 to 12 months without neuroleptic
medication, compared with 10 to 30% who relapse when typical neuroleptics are
maintained. Long-term outcome studies showed that persistent symptoms that do
not respond to standard neuroleptic therapy are associated with a greater risk
of rehospitalization. Nonpharmacological interven-tions may help decrease
relapse rates (discussed later).
Long-term treatment of schizophrenia is a complex issue. It is clear
that the majority of patients require maintenance medi-cation. Some patients do
well with stable doses of neuroleptics for years without any exacerbations.
However, many patients who are maintained with a stable neuroleptic dose have
episodic breakthroughs of their psychotic symptoms. The difficulty in
tol-erating neuroleptic side effects often results in noncompliance with
medication. It is therefore prudent to assess patients for medication
compliance when signs of relapse are suspected. Pro-dromal cues may be present
before an exacerbation of psychotic symptoms. For example, any recent change in
sleep, attention to activities of daily living, or disorganization may be a
warning sign of an impending increase in psychosis.
For patients for whom compliance is a problem, long-act-ing, depot
neuroleptics are available in the USA for both tradi-tional and novel
antipsychotics. This form of medication deliv-ery guarantees that the
medication is in the system of the person taking it and eliminates the need to
monitor daily compliance. This alternative should be considered if
noncompliance with oral agents has led to relapses and rehospitalization. With
these patients, maintenance treatment using long-acting preparations should begin
as early as possible. Depot antipsychotic drugs are effective maintenance
therapy for patients with schizophrenia.
Many
studies have investigated appropriate maintenance doses of standard
antipsychotics. Effective maintenance treatment is defined as that which
prevents or minimizes the risks of symp-tom exacerbation and subsequent
morbidity. A series of interest-ing dose-finding studies were performed by Kane
and colleagues (1983, 1985) to determine the minimal dosage required to prevent
relapse and to reduce the risk of extrapyramidal symptoms and tardive
dyskinesia. This group found that the relapse rate (56%) of patients treated
with lower doses of fluphenazine decanoate (1.25– 5 mg every 2 weeks) was
significantly greater than the relapse rate (14%) of patients receiving
standard doses (12.5–50 mg every 2 weeks). Other investigators have found that
this low dosage range may appear to prevent relapse for a certain period
(Marder et al., 1984)
but fails to do so if patients are followed up for more than 1 year (Marder et al., 1987).
Unfortunately, no specific dosage reliably prevents relapse, and there is no
way to predict future relapse. This is true for the novel antipsychotic agents
as well.
Related Topics