Chapter: Essentials of Psychiatry: Schizophrenia and Other Psychoses
Schizophrenia: Neuroanatomical Theories
Neuroanatomical Theories
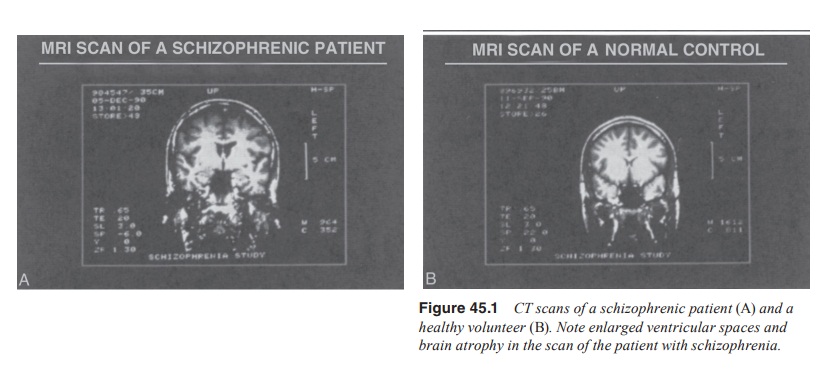
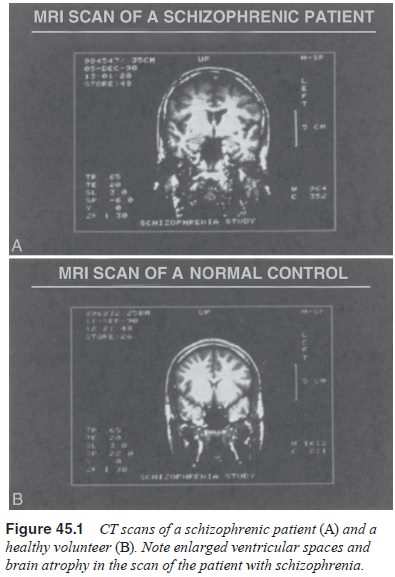
Enlarged Ventricles
The ventricles are the fluid-filled spaces in the center of the brain.
The most consistent morphological finding in the literature of schizophrenia is
enlarged ventricles which has been confirmed by a large number of CT and MRI
studies. The effect size of ven-triculomegaly has been reported to be 0.7 (Raz
and Raz, 1990). Seventy-nine percent of the well designed studies report
enlarge-ment of lateral ventricles. Lawrie and Abukmeil (1998) report
approximately 40% difference in volume between schizophrenia patients and
controls across all volumetric MRI studies. It should be noted that although
the ventricular increases are statistically significant, the ventricles are not
grossly enlarged in most cases. In fact, radiologists most often read CT and
MRI scans of patients with schizophrenia as normal. In addition, most studies
of ven-tricular size demonstrate overlap between patients and normal control
subjects, indicating that many patients have ventricles in the normal range.
Nonetheless, enlargement of the ventricles is the first consistently reported
finding confirming a brain abnor-mality in schizophrenia.
The pathophysiological significance of larger than normal ventricles is
unclear. Enlarged ventricles are most likely a secon-dary manifestation of
brain atrophy or some other process resulting in either focal or generalized
reductions in brain mass. Indeed, there have been many reports of brain atrophy
and reduced mass in the illness (Figure 45.1). Enlarged ventricles have also
been reported in first-degree relatives of subjects with schizophrenia (Cannon et al., 1998; Seidman et al., 1997) and in persons suffering
from schizotypal personality disorder (Buchsbaum et al., 1997) raising interesting speculations of whether
ventriculomegaly may be an indicator of neurodevelopmental risk for
schizophrenia (Lencz et al., 2001).
Limbic System
The limbic structures that have been implicated in schizophrenia are the
hippocampus, entorhinal cortex, anterior cingulate and amygdala. These
structures have important functions for memory (hippocampus), attention
(anterior cingulate), and emotional ex-pression and social affiliation
(amygdala). The entorhinal cortex serves as a “way station” between hippocampus
and neocortex in that neurotransmissions between these regions synapse in the
entorhinal cortex. The entorhinal cortex, hippocampus and other components of
the parahippocampal gyrus are often considered “mesiotemporal” structures
because of their close anatomical and functional relationship.
There are more reports of abnormalities in hippocampal and related mesiotemporal structures than other limbic struc-tures in schizophrenia. In fact, mesiotemporal pathology is con-sistently found in studies of schizophrenia and mesiotemporal structures are leading candidates for the neuroanatomical site of this condition. This region has been implicated by converging brain imaging and postmortem lines of evidence. One of the most consistent MRI morphological findings is reduction in size of the hippocampus. In addition, more than 25 postmortem studies have reported morphological and cytoarchitectural abnormalities in this structure. The findings have included reduced size and cellular number (white matter reductions, and abnormal cell ar-rangement. There is a bilateral reduction of approximately 4% hippocampal volume in schizophrenia. However, reduced hip-pocampal volume is not reported by all studies.
The anterior cingulate has been implicated in schizophre-nia largely
because of postmortem findings of reduced gamma-aminobutyric acid (GABA)
interneurons. In addition, functional imaging studies have demonstrated altered
metabolic activity both at rest and during selective attention tasks in the
anterior cingulate in patients with schizophrenia. Thirty-one studies evaluated
one or more of the medial temporal lobe structures – hippocampus, amygdala,
parahippocampal gyrus, entorhinal cortex – with 77% reporting positive
findings; this is one of the higher percentages of abnormalities reported in
all regions of interest throughout the brain.
Prefrontal Cortex
The prefrontal cortex is the most anterior portion of the neocor-tex,
sitting behind the forehead. It has evolved through lower spe-cies to become
one of the largest regions of the human brain, constituting approximately
one-third of the cortex. It is respon-sible for some of the most sophisticated
human functions. It contains a heteromodal association area that is responsible
for integrating information from all other cortical areas as well as from several
subcortical regions for the execution of purpose-ful behavior. Among its
specific functions are working mem-ory, which involves the temporary storage
(seconds to minutes) of information, attention and suppression of interference
from internal and external sources. The most inferior portion of the prefrontal
cortex, termed the orbital frontal cortex, is involved in emotional expression.
Given its unique role, it is not surprising that the prefrontal cortex has been
considered in the etiology of schizophrenia.
Indeed,
several lines of evidence have implicated the pre-frontal cortex in
schizophrenia. CT studies have provided evi-dence for prefrontal atrophy, and
some, although not all, MRI studies have found evidence for decreased volume of
this struc-ture. One of the earliest observations from functional imaging
studies of schizophrenia was reduced perfusion of the frontal lobes. This
finding was subsequently replicated by several PET studies suggesting decreased
frontal glucose utilization and blood flow, which came to be known as hypofrontality.
Subsequent functional imaging studies provided further support for
hypof-rontality by demonstrating that patients with schizophrenia failed to
activate their frontal lobes to the same degree as normal control subjects when
performing frontal cognitive tasks. This finding has been questioned because
patients with schizophrenia typi-cally perform poorly in many cognitive
paradigms, so it is unclear whether their lack of frontal activation is a
primary frontal deficit or secondary to poor cognitive task performance related
to factors such as lack of motivation, inattention, or cognitive impairment
stemming from nonfrontal regions. Auditory hallucinations were found to be
associated with increases in Broca’s area, a portion of the frontal cortex
responsible for language production. This find-ing was of interest because it
supported a hypothesis that auditory hallucinations were a form of abnormal
“inner speech”.
MRI studies employing diffusion tensor imaging have re-ported changes
suggestive of an abnormality in white matter con-nectivity possibly due to
reduced myelination of fiber tracts in patients with schizophrenia (Buchsbaum et al., 1998). Magnetic resonance
spectroscopy (MRS) studies have reported reduced levels of neuronal membrane
constituents (phosphomonoesters) and/or increased levels of their breakdown
products (phosphodi-esters) in patients with schizophrenia, primarily in the
frontal cortex. Such abnormalities have been observed in treatment-na-ive first
episode patients and have been correlated with trait-like negative symptoms and
neurocognitive performance.
Though sometimes contradictory, the neuroimaging stud-ies consistently
report abnormalities in the orbitofrontal region; often, these abnormalities tend
to correlate with severity of schizophrenia symptomatology, show gender
differences in rela-tion to spatial localization and the gray matter deficits
may be more widespread in chronic, as compared with medication-naive first
episode patients. Additional support for prefrontal cortical involvement in
schizophrenia comes from postmortem studies with a range of findings. There
have been reports of reduced cortical thickness, loss of pyramidal cells,
malformed cellular architecture, loss of GABA interneurons and evidence of
failed neuronal migration. A majority of the abnormalities represented a
decline in function suggesting a widespread failure of gene ex-pression.
Specifically, abnormalities involving the glycoprotein Reelin were observed in schizophrenia, a finding reported previ-ously by other postmortem studies. Reelin, an extracellular ma-trix
glycoprotein secreted from different GABAergic interneu-rons during development
and adult life, may be important for the transcription of specific genes
necessary for synaptic plasticity and morphological changes associated with
learning.
Temporal Lobe
The superior temporal gyrus is involved in auditory processing and, with
parts of the inferior parietal cortex, is a heteromodalassociation area that
includes Wernicke’s area, a language center. Because of the important role it
plays in audition, it was hypoth-esized to be involved in auditory
hallucinations. Indeed, MRI studies have found the superior temporal gyrus to
be reduced in size in schizophrenia and have found a significant relation-ship
between these reductions and the presence of auditory hal-lucinations.
Similarly, Wernicke’s area, which is involved in the conception and
organization of speech, has been hypothesized to mediate the thought disorder
of schizophrenia, particularly conceptual disorganization. Support for this
hypothesis comes from a report of a patient with vascular and other lesions of
this region that produce Wernicke’s aphasia, a disruption in the or-ganization
of speech that resembles the thought disorder of schizophrenia. MRI studies
have found a relationship between morphological abnormalities in this region
and conceptual dis-organization in schizophrenia. McCarley and colleagues
(1999) reviewed 118 MRI studies published from 1988 to 1998; 62% of the 37
studies of whole temporal lobe showed volume reduction and/or abnormal
asymmetry especially in the superior temporal gyrus, the highest percentage of
any cortical region of interest.
Striatum
The striatum, consisting of the caudate, putamen, globus pal-lidus,
substantia nigra and accumbens, is an output center for the cortex and has been
traditionally thought to have a primary role in the execution of motor
programs. Subsequent studies have demonstrated an important cognitive role for
this structure as well. Moreover, in primary diseases of the striatum, such as
Parkinson’s and Huntington’s diseases, clinical manifestations include
psychosis and other schizophrenia type behavior, which has contributed to
interest in this region in the pathophysiology of schizophrenia.
Two related bodies of data are most frequently cited re-garding the role
of the striatum in schizophrenia; these concern the mechanism of antipsychotic
drugs and postmortem studies of altered dopamine D2 receptor numbers. The dorsal
striatum (caudate and putamen) is the site of the vast majority of D2 re-ceptors in the brain. All
effective antipsychotic drugs antagonize this receptor and thus, by
extrapolation, it was reasoned that this region might be central to the pathophysiology
of schizophrenia. Moreover, the most consistent postmortem finding in the
schizo-phrenia literature is an increased density of striatal D2 receptors. However, neuroleptic
exposure causes up-regulation of D2 recep-tors, which may account for this postmortem finding. A current
view of the antipsychotic mechanism is that the dorsal striatum is involved in
mediating the extrapyramidal side effects of antipsy-chotic medications and,
based on rodent studies of antipsychotic drug mechanisms, the ventral striatum
(nucleus accumbens) may be involved in antipsychotic efficacy. Thus, attention
has shifted toward the possible role of the accumbens in mediating the
psy-chosis of schizophrenia.
Thalamus
The thalamus is a nucleus that receives subcortical input and out-puts
it to the cortex. One theory posits that the thalamus provides a filtering
function for sensory input to the cortex. A deficit in thalamic filtering was
proposed to account in part for the experi-ential phenomena of being
overwhelmed by sensory stimuli re-ported by many patients with schizophrenia.
Preclinical studies have demonstrated that antipsychotic drugs modulate
thalamic input to the cortex, which has been offered as a model for
antip-sychotic drug action. Several MRI studies have reported reduced volume,
and functional abnormalities of the thalamus in patients with schizophrenia.
Postmortem studies have also found cell loss and reductions in tissue volume in
thalamic nuclei. This thalamic tissue reduction is considered as a possible evidence
of abnormal circuitry linking the cortex, thalamus and cerebellum.
Neural Circuits
Because of the large number of different neuroanatomical find-ings in
studies of schizophrenia and the appreciation that brain function involves
integration of several brain regions, current thinking about the neuroanatomy
of this illness is centered on neural circuits. It is conceivable that an
isolated lesion anywhere in a neural circuit could result in dysfunction of the
entire net-work, and therefore spurious conclusions could be drawn by
in-vestigating only one component of a neural network. Evidence suggests that
schizophrenia may be associated with a decrease in synaptic connectivity of the
dorsal prefrontal cortex though this is not reported by all studies. McGlashan
and Hoffman (2000) have proposed the Developmentally Reduced Synaptic
Connec-tivity (DRSC) model which proposes that cortical gray matter deficits
may arise from either reduced baseline synaptic density due to genetic and/or
perinatal factors, or excessive pruning of synapses during adolescence and
early adulthood or both. There is regionally specific decreased neuronal size
in cortical layer III with cytoplasmic atrophy and generally reduced neuropil.
The reduced size and increased density of neurons or glia and de-creased
cortical thickness suggest that cell processes and synap-tic connections are
reduced in schizophrenia. This is consistent with reports of decreased
concentrations of synaptic proteins (e.g., synaptophysin). These cell processes
and synapses could be lost as a consequence of a neurochemically mediated
(through dopamine and or glutamate) synaptic apoptosis that would com-promise
cell function and alter brain morphology without, how-ever, producing serious
cell injury (and thus inducing glial reac-tions). However, McCarley and
colleagues (1999) suggest that the main neural abnormality in schizophrenia
involves neural con-nectivity (dendrite/neuropil/gray matter changes) rather
than the number of neurons or network size. They suggest that a “failure of
inhibition” on the cellular level is present in schizophrenia and may be linked
to a “failure of inhibition” at the cognitive level. According to Lafargue and
Brasic (2000) abnormalities involv-ing the temporolimbic–prefrontal cerebral
circuitry is postulated to underlie the organizational and memory deficits
commonly observed in schizophrenia patients. Furthermore, as reviewed by these
authors, a possible insult or injury to the mechanism of GABAergic and
glutamatergic influence during early corticogen-esis may largely contribute to
the later manifestation of clinical schizophrenia. Malfunction of the
cooperating sensory systems of excitation and inhibition during the early
stages of develop-ment of the brain could result in the failure of “pioneer
neurons” properly to differentiate and migrate to their appropriate cerebral
locations. Consequently the later migrating projection neurons may fail to
reach or invade their preselected area-specific brain sites. A disturbance of
the proper GABAergic and glutamatergic influences would upset NMDA mechanisms
and normal corti-cal development. If such disturbance is actively occurring
from the onset of cerebral ontogeny, the affected individual may suf-fer from
the signs and symptoms observed in schizophrenia. A challenge for the future is
developing new approaches to examin-ing the brain as an integrated and highly
interactive system. An unanswered question is whether the morphological
differences reflect hypoplasia (failure to develop) or atrophy (shrinkage)
Electrophysiology
Electroencephalogram (EEG) records the electrical activity of brain,
which may reflect the mental functions carried out by the neurons possibly in
“real time”. However, the precise localization of this event in the specific
brain region is poor. When EEG activ-ity from repeated presentations of a
specific stimulus is summed across trials, some potentials related to the
specific processing of the target stimulus can be extracted from the EEG and
are referred as event-related potentials
(ERPs).
The P300 ERP, a positive deflection occurring approxi-mately 300
milliseconds after the introduction of a stimulus, is regarded as a putative
biological marker of risk for schizophrenia. The P300 amplitudes are smaller in
patients with schizophrenia and is one of the most replicated
electrophysiological findings.
Related Topics