Chapter: Clinical Anesthesiology: Perioperative & Critical Care Medicine: Postanesthesia Care
Routine Recovery
ROUTINE RECOVERY
General Anesthesia
Airway patency, vital signs, oxygenation, and
level of consciousness must be assessed immediately upon PACU arrival.
Subsequent blood pressure, heart rate, and respiratory rate measurements are
routinely made at least every 5 min for 15 min or until stable, and every 15
min thereafter. Pulse oximetry should be monitored continuously in all
patients. The occurrence of hypoxemia does not necessarily correlate with the
level of consciousness. Neuromuscular function should be assessed clini-cally
(eg, head-lift and grip strength). At least one temperature measurement must
also be obtained. Additional monitoring includes pain assessment (eg, numerical
or descriptive scales); the presence or absence of nausea or vomiting; and
fluid input and output, including urine flow, drainage, and bleed-ing. After
initial vital signs have been recorded, the anesthesia provider should give a
brief report to the PACU nurse that includes (1) the preoperative his-tory
(including mental status and any communica-tion problems, such as language
barriers, deafness, blindness, or mental disability); (2) pertinent
intra-operative events (type of anesthesia, the surgical procedure, blood loss,
fluid replacement, antibiotic and other relevant medication administration, and
any complications); (3) expected postoperative problems; (4) anticipated need
for PACU medica-tion administration, such as antibiotics; and (5)
postanesthesia orders (analgesia and nausea/vom-iting therapy; epidural or
perineural catheter care; including the need for acute pain service
involve-ment, administration of fluids or blood products, postoperative
ventilation, chest x-ray for follow-up of central venous catheterization,
etc.).All patients recovering from general anesthe-sia must receive
supplemental oxygen and pulse oximetry monitoring during emergence because
transient hypoxemia can develop even in healthy patients. A rational decision
regarding continuation of supplemental oxygen therapy at the time of
PACUdischarge can be made based on Spo2 readings on room air. Arterial blood gas measurements maybe obtained to
confirm abnormal oximetry read-ings, but are not necessary in most patients.
Oxygen therapy should be carefully controlled in patients with chronic
obstructive pulmonary disease and ahistory of, or potential for, CO 2
retention. Patients should generally be nursed in the back-up position,
whenever possible, to optimize oxygenation. However, elevating the head of the
bed before the patient is responsive can lead to airway obstruction. In such
cases, the oral or nasal airway should be left in place until the patient is
awake and able to main-tain airway. Deep breathing and coughing should be
encouraged periodically.
Regional Anesthesia
Patients who are heavily sedated or
hemodynami-cally unstable following regional anesthesia should also receive supplemental
oxygen in the PACU. Sensory and motor levels should be periodically recorded
following regional anesthesia to document regression of the block. Precautions
in the form of padding or repeated warning may be necessary to prevent
self-injury from uncoordinated arm move-ments following brachial plexus blocks.
Blood pres-sure should be closely monitored following spinal and epidural
anesthesia. Bladder catheterization may be necessary in patients who have had
spinal or epidural anesthesia for longer than 4 hr.
Pain Control
Moderate to severe postoperative pain is most
commonly treated with oral or parenteral opioids. However, perioperative opioid
administration is associated with side effects (nausea and vomit-ing,
respiratory depression, pruritis, ileus, and uri-nary retention) which may have
significant adverse effects on postoperative convalescence. In response to this
problem, a variety of opioid sparing
strategies have been increasingly embraced over the past two decades to
decrease opioid requirements, and thus opioid-related side effects, while
maintaining satis-factory analgesia . Preoperative oral administration of
nonsteroidal antiinflammatory drugs (NSAIDs), acetaminophen, and gabapentin or
pregabalin may significantly reduce postoperative opioid requirements, and
these medications may be resumed postoperatively if the patient can continue
oral medication. Additional analgesic modalities utilizing local anesthetics,
such as intraoperative wound infiltration, postoperative wound catheter infusions,
single-shot and continuous catheter peripheral nerve blocks, and continuous
epidural infusions, also reduce postoperative opioid analgesic requirements,
and thus also reduce opioid-related side effects.
Mild to moderate postoperative pain can be treated orally with
acetaminophen, ibuprofen, hydrocodone, or oxycodone. Alternatively, ketorolac
tromethamine (15–30 mg in adults) or acetamino-phen (15 mg/kg, or 1 g if
patient >50 kg) may
be administered intravenously.
Insituations where moderate to severe
postop-erative pain is present, or oral analgesia is not possi-ble, parenteral
or intraspinal opioids, single-shot or continuous nerve blocks, and continuous
epidural analgesia are used, often in combination techniques. Parenteral
opioids are most safely administered by titration of small doses. Considerable
variability in opioid requirements should be expected in surgi-cal patients
recovering in the PACU, and adequate analgesia must be balanced against the
risk of exces-sive sedation and respiratory depression. Opioids of intermediate
to long duration, such as hydro-morphone 0.25–0.5 mg (0.015–0.02 mg/kg in
chil-dren) or morphine 2–4 mg (0.025–0.05 mg/kg in children), are most commonly
used. Meperidine is most often used in small doses to treat postoperative
shivering. Opioid requirements are often markedly increased in patients with a
history of chronic pain and chronic opioid therapy, because of opioid
toler-ance, and in patients with a history of opioid addic-tion, because of
opioid tolerance and psychological dependence. Consultation with a pain
specialist is often extremely helpful in these situations.
Analgesic effects of parenteral opioids usually peak within minutes of administration.
Maximal respiratory depression, particularly with morphine and hydromorphone,
may not occur until 20–30 min later. When the patient is fully awake,
patient-controlled analgesia can be instituted for inpatients. Intramuscular
administration of opioids is discour-aged because delayed and variable onset
(10–20 min or longer) and delayed respiratory depression (up to 1 h).
When an epidural catheter is used, epidural
bolus administration of fentanyl (50–100 mcg) or sufentanil (20–30 mcg) with
5–10 mL of 0.1% bupi-vacaine can provide excellent pain relief in adults.
Epidural morphine (3–5 mg) may also be used, but delayed respiratory depression
with epidural admin-istration of this opioid mandates close monitoring for 24
hr afterward .
Agitation
Before the recovering patient is fully
respon-sive, pain is often manifested as postoperativerestlessness. Serious
systemic disturbances (such as hypoxemia, respiratory or metabolic acidosis, or
hypotension), bladder distention, or a surgical complication (such as occult
intraabdominal hem-orrhage) must also be considered in the differential
diagnosis of postoperative agitation. Marked agita-tion may necessitate arm and
leg restraints to avoid self-injury, particularly in children. When serious
physiological disturbances have been excluded in children, cuddling and kind
words from a sympa-thetic attendant or the parents often calms the pedi-atric
patient. Other contributory factors include marked preoperative anxiety and
fear, as well as adverse drug effects (large doses of central anticho-linergic
agents, phenothiazines, or ketamine). Phy-sostigmine 1–2 mg intravenously (0.05
mg/kg in children) is most effective in treating delirium due to atropine and
scopolamine. If serious systemic disturbances and pain are excluded, persistent
agita-tion may require sedation with intermittent intrave-nous doses of
midazolam 0.5–1 mg (0.05 mg/kg in children).
Nausea & Vomiting
Postoperative nausea and vomiting (PONV) is
com-mon following general anesthesia, occurring in 30% to 40% of all patients.
Moreover, PONV occurs at
home within 24 hr of an uneventful discharge
(post-discharge nausea and vomiting) in a significant number of ambulatory
surgery patients. The etiol-ogy of PONV is usually multifactorial and
associ-ated with anesthetic and analgesic agents, the type of surgical
procedure, and intrinsic patient factors, such as a history of motion sickness.
It is also impor-tant to recognize that nausea is a common complaint reported at
the onset of hypotension, particularly following spinal or epidural anesthesia.
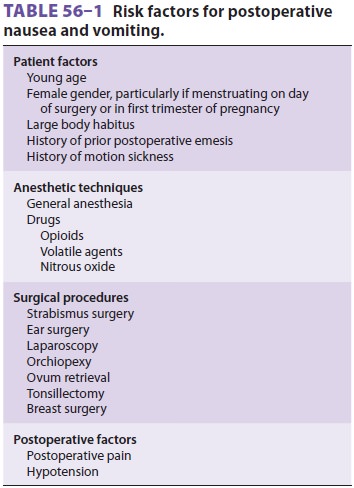
Table
56–1 lists commonly
recognized riskfactors for PONV. An increasedincidenceofnauseaand vomiting is
reported following opioid adminis-tration and intraperitoneal (especially
laparoscopic), breast, and strabismus surgery. The greatest inci-dence seems to
be in young women; nausea may be more common during menstruation. Increased
vagal tone manifested as sudden bradycardia com-monly precedes, or coincides
with, emesis. Propo-fol anesthesia decreases the incidence of PONV, and a
preoperative history of smoking lessens the likelihood of PONV. Selective
5-hydroxytryptamine
(serotonin) receptor 3 (5-HT 3)
antagonists, such as ondansetron 4 mg (0.1 mg/kg in children), granisetron 0.01–0.04
mg/kg, and dolasetron 12.5 mg (0.035 mg/kg in children), are effective in
preventing PONV, and, to a lesser extent, in treating established PONV. It
should be noted that unlike ondansetron, which is usually effective
immediately, dolasetron requires 15 min for onset. An orally disintegrating
tablet preparation of ondansetron (8 mg) may be useful for treatment and
prophylaxis against post-discharge nausea and vomiting. Metoclopramide, 0.15
mg/kg intravenously, is a less effective alternative to 5-HT3
antagonists. 5-HT3 antagonists are not associated with the acute
extrapyramidal (dystonic)manifestations and dysphoric reactions that may be
encountered with metoclopramide or phenothi-azine-type antiemetics. Transdermal
scopolamine is effective, but can be associated with side effects, such as
sedation, dysphoria, blurred vision, dry mouth, urinary retention, and
exacerbation of glaucoma, particularly in elderly patients. Dexamethasone 4–10
mg (0.10 mg/kg in children), when utilized as an antiemetic, has the additional
advantages of pro-viding a varying degree of analgesia and a sense of patient
well-being. Moreover, it seems to be effec-tive for up to 24 hr, and, thus, may
be useful for postdischarge nausea and vomiting. Oral aprepitant (Emend®) 40 mg
may be administered within 3 hr prior to anesthesia induction. Intravenous
droperi-dol 0.625–1.25 mg (0.05–0.075 mg/kg in children), when given
intraoperatively, significantly decreases the likelihood of PONV.
Unfortunately, droperidol carries a US Food and Drug Administration “black box”
warning, indicating that large (5–15 mg) doses can prolong the QT interval and
have been associ-ated with fatal cardiac arrhythmias. Nonpharmaco-logical
prophylaxis against PONV includes ensuring adequate hydration (20 mL/kg) after
fasting, and stimulation of the P6 acupuncture point (wrist). The latter may
include application of pressure, electrical current, or injections.
Controversy exists regarding routine PONV
prophylaxis for all patients. Because of the cost of treatment of established
PONV, it may be cost-effective to provide prophylaxis to all patients in
cer-tain populations (eg, outpatients). Clearly, patients with multiple risk
factors should receive prophy-laxis. In addition, the use of two or three
agents that act on differing receptors is more
effective than sin-gle-agent prophylaxis.
Shivering & Hypothermia
Shivering can occur in the PACU as a result
of intra-operative hypothermia or the effects of anesthetic agents, and it is
also common in the immediate post-partum period. The most important cause of
hypo-thermia is a redistribution of heat from the body core to the peripheral
compartments. A relatively cool ambient operating room temperature, prolonged
exposure of a large wound, and the use of large amounts of unwarmed intravenous
fluids or high flows of unhumidified gases can also be con-tributory. Nearly
all anesthetics, particularly volatile agents and spinal and epidural
anesthesia, decrease the normal vasoconstrictive response to hypother-mia by
decreasing sympathetic tone. Although anes-thetic agents also decrease the
shivering threshold, shivering commonly observed during or after emer-gence
from general anesthesia represents the body’s effort to increase heat
production and raise body temperature and may be associated with intense
vasoconstriction. Emergence from even brief gen-eral anesthesia is sometimes
also associated with shivering, and although the shivering can be one of
several nonspecific neurological signs (postur-ing, clonus, or Babinski’s sign)
that are sometimes observed during emergence, it is most often due to
hypothermia. Regardless of the mechanism, its inci-dence seems to be related to
the duration of surgery and the use of a volatile agent. Shivering may
occa-sionally be sufficiently intense to cause hyperther-mia (38–39°C) and significant
metabolic acidosis, both of which promptly resolve when the shivering stops.
Other causes of shivering should be excluded, such as bacteremia and sepsis,
drug allergy, or trans-fusion reaction.
Hypothermia should be treated with a
forced-air warming device, or (less satisfactorily) with warming lights or
heating blankets, to raise body temperatureto normal. Intense shivering causes
precipitous rises in oxygen consumption, CO2 production,and cardiac
output. These physiological effects are often poorly tolerated by patients with
pre-existing cardiac or pulmonary impairment. Hypothermia has been associated
with an increased incidence of myo-cardial ischemia, arrhythmias, increased
transfusion requirements due to coagulopathy, and increased duration of muscle
relaxant effects. Small intravenous doses of meperidine (10–25 mg) can
dramatically reduce or even stop shivering. Intubated and mechan-ically
ventilated patients can also be sedated and given a muscle relaxant until
normothermia is rees-tablished by active rewarming and the effects of
anes-thesia have dissipated.
Discharge Criteria
A. PACU
All patients must be evaluated by a qualified anes-thesia provider prior
to discharge from the PACU unless strict discharge criteria are adopted.
Stan-dards for discharging patients from the PACU are established by the
department of anesthesiology and the hospital medical staff. They may allow
PACU nurses to determine when patients may be trans-ferred without the presence
of a qualified anes-thesia provider if all PACU discharge criteria have been
met. Criteria can vary according to whether the patient is going to be
discharged to an intensive care unit, a regular ward, the outpatient department
(phase 2 recovery), or directly home.
Before discharge, patients should have been observed for respiratory
depression for at least 20–30 min after the last dose of parenteral opioid.
Other minimum discharge criteria for patients recovering from general
anesthesia usually include the following:
·
Easy arousability
·
Full orientation
·
The ability to maintain and protect
the airway
·
Stable vital signs for at least 15–30
min
·
The ability to call for help, if
necessary
·
No obvious surgical complications
(such as active bleeding)
Postoperative pain and nausea and vomiting
must be controlled, and normothermia should be reestablished prior
to PACU discharge. Scoring sys-tems are widely used. Most assess Spo2
(or color),
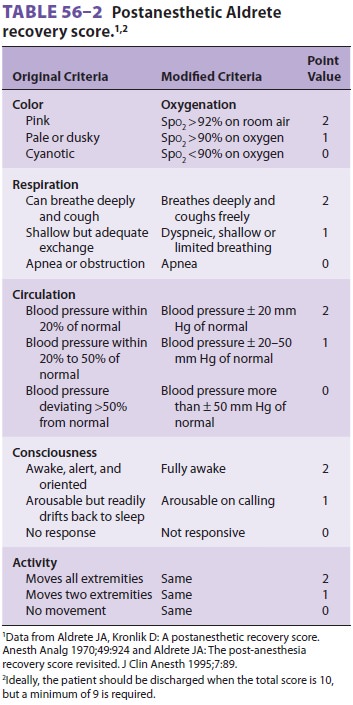
consciousness, circulation, respiration, and motor
activity (Table
56–2). The majority of
patients can meet discharge criteria within 60 min from the time of PACU
arrival. Patients to be transferred to other intensive care areas need not meet
all requirements.
In addition to the above criteria, patients receiv-ing regional
anesthesia should also be assessed for regression of both sensory and motor
block-ade. Complete resolution of the block prior to PACU dismissal avoids
inadvertent injuries due to motor weakness or sensory deficits; however,
many
institutions have protocols that allow earlier discharge to appropriately
monitored areas, and patients may be discharged with peripheral nerve blocks
from single-shot or continuous perineural catheter infusions for the purpose of
regional anal-gesia. Documenting regression of a block is impor-tant. Failure
of a spinal or epidural block to resolve 6 hr after the last dose of local
anesthetic raises the possibility of spinal subdural or epidural hematoma,
which should be excluded by prompt radiological imaging and neurologic
evaluation.
In some
centers, outpatients who meet the above discharge criteria when they come out
of the operating room may be “fast-tracked,” bypassing the PACU and proceeding
directly to the phase 2 recov-ery area. Similarly, inpatients who meet the same
criteria may be transferred directly from the operat-ing room to their ward, if
appropriate staffing and monitoring is present.
B. Outpatients
In addition to emergence and awakening,
recovery from anesthesia following outpatient procedures includes two
additional stages: home readiness (phase 2 recovery) and complete psychomotor
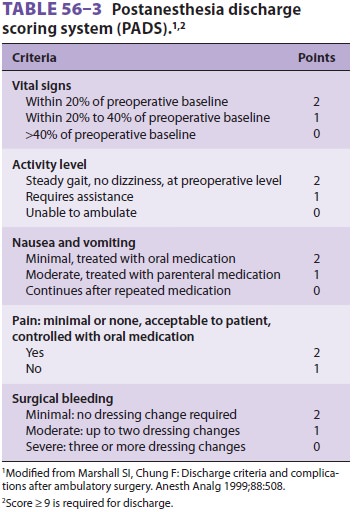
recovery. A scoring system has been developed to help assess home readiness
discharge (Table 56–3). Recovery of
proprioception, sympathetic tone, blad-der function, and motor strength are
additional crite-ria following regional anesthesia. For example, intact
proprioception of the big toe, minimal orthostatic blood pressure and heart
rate changes, and normal plantar flexion of the foot are important signals of
recovery following spinal anesthesia. Urination and drinking or eating before
discharge are usually no longer required; exceptions include patients with a
history of urinary retention and those with diabetes.
All outpatients must be discharged home in
the company of a responsible adult who will stay with them overnight (the
latter is required if they have received an anesthetic). Patients must be
provided with written postoperative instructions on how to obtain emergency
help and to perform routine follow-up care. The assessment of home readiness is
the responsibility of the qualified anesthesia pro-vider, preferably one who is
already familiar with the patient, although authority to discharge a patient
home can be delegated to a nurse, if approved dis-charge criteria are
applied.
Home readiness does not imply that the patient has the ability to make
important decisions, to drive, or to return to work. These activities require
complete psychomotor recovery, which is often not achieved until 24–72 hr
postoperatively. All outpa-tient centers must use some system of postoperative
follow-up, preferably phone contact the day after discharge.
Related Topics