Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Rickettsia, Coxiella, Ehrlichia, and Bartonella
Rickettsial Disease : Clinical Aspects
RICKETTSIAL DISEASE : CLINICAL ASPECTS
SPOTTED FEVER GROUP
The most important rickettsial disease in North America is RMSF, which is caused by Rickettsia rickettsii. A number of other spotted fever rickettsioses are found in otherparts of the world (see Table 31–1); the name often reveals the locale (eg, Mediter-ranean spotted fever, Marseilles fever). They are caused by Rickettsia conorii, a species serologically related to, but distinct from, R. rickettsii. Another less severe spotted fever, rickettsialpox, also occurs in North America.
Rocky Mountain Spotted Fever
RMSF is an acute febrile illness that occurs in association with residential and recre-ational exposure to wooded areas where infected ticks exist. The disease has a significant mortality (25%) if untreated.
Epidemiology
R. rickettsii is primarily a parasite of ticks. In the western United States, the wood tick(Dermacentor andersoni) is the primary vector. In the East, the dog tick (Dermacentorvariabilis) is the natural carrier and vector of the disease, and in the Southwest and Mid-west, the vector is the Lone Star tick (Amblyomma americanum). R. rickettsii does not kill its arthropod host, so the parasite is passed through unending generations of ticks by transovarial spread. Adult females require a blood meal to lay eggs and thus may transmit the disease. Infected adult ticks have been shown to survive as long as 4 years without feeding.
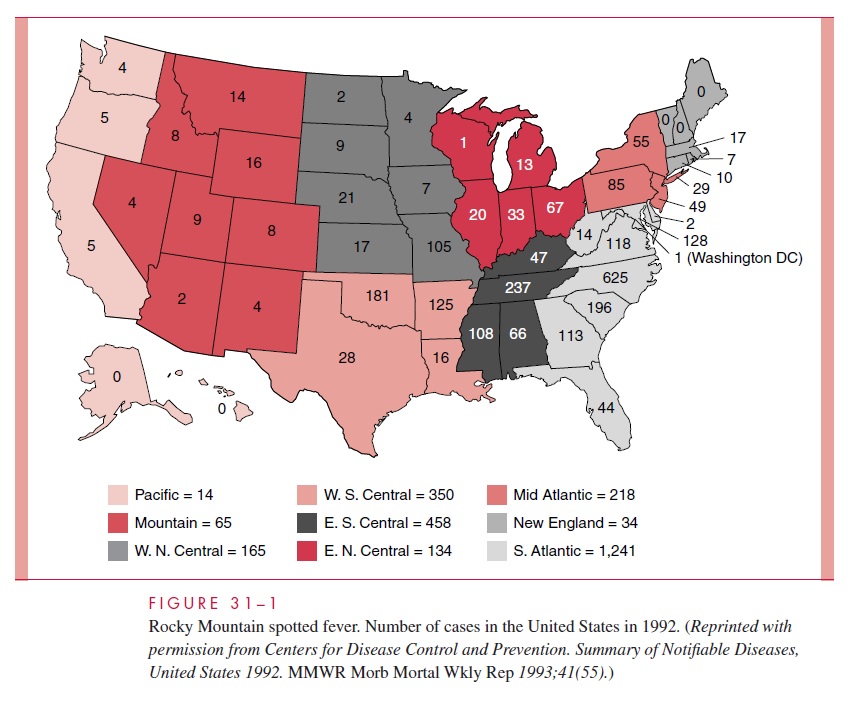
R. rickettsii is found in both North America and South America. The highest attackrates in the United States are in the central and mid-Atlantic states (Fig 31–1). The US in-cidence increased in the 1970s and early 1980s to more than 0.5 cases per 100,000 popu-lation but has since decreased to less than half that figure. More than two thirds of cases are in children less than 15 years of age. The illness is generally seen between April and September because of increased exposure to ticks. A history of tick bite can be elicited in approximately 70% of cases.
Manifestations
The incubation period between the tick bite and the onset of illness is usually 2 to 6 days but may be as long as 2 weeks. Fever, headache, rash, toxicity, mental confusion, and myalgia are the major clinical features. The rash is the most characteristic feature of the illness. It usually develops on the second or third day of illness as small erythematous macules that rapidly become petechial.
The lesions appear initially on the wrists and an-kles and then spread up the extremities to the trunk in a few hours. A diagnostic feature of RMSF is the frequent appearance of the rash on the palms and soles, a finding not usually seen in maculopapular eruptions associated with viral infections. Muscle tenderness, especially in the gastrocnemius, is characteristic and may be extreme. If untreated, or in occasional cases despite therapy, complications such as disseminated intravascular coagu- lation, thrombocytopenia, encephalitis, vascular collapse, and renal and heart failure may ensue.
Diagnosis
Because serologic testing is the primary diagnostic approach, it is often difficult to estab-lish the diagnosis of RMSF early in the course of illness. However, antibodies may appear by the sixth or seventh day of illness, and a fourfold rise in antibody titer between acute serum and convalescent serum establishes the diagnosis. Specific therapy must usually be started solely on the basis of clinical signs, symptoms, and epidemiologic considerations.
Treatment
Appropriate antibiotic therapy is highly effective if given during the first week of illness. If delayed into the second week or when pathologic processes such as disseminated intravas-cular coagulation are present, therapy is much less effective. The antibiotic of choice is doxycycline. Sulfonamides may worsen the disease process and are thus contraindicated. Before specific therapy became available, the mortality of RMSF was approximately 25%. Treatment has reduced this figure to between 5 and 7%. Death results primarily in patients in whom diagnosis and therapy are delayed into the second week of illness.
Prevention
The major means of preventing RMSF is avoidance or reduction of tick contact. Frequent deticking in tick-infested areas is important, because ticks generally must feed for 6 hours or longer before they can transmit the disease. Tick surveys in the Carolinas have shown infection in about 5% of samples. Killed vaccines prepared from infected ticks, or rick-ettsias grown in embryonated eggs and cell cultures have been developed. None is licensed for clinical use at present.
Rickettsialpox
Rickettsialpox was first recognized in New York City in 1946. It is a benign rickettsial ill-ness caused by Rickettsia akari and transmitted by a rodent mite. Distinguishing features of the disease include an eschar at the site of the bite and a vesicular rash. The house mouse and other semidomestic rodents are the primary reservoir. Humans acquire infec-tion when the mite seeks an alternative host.
Rickettsialpox is a biphasic illness. The first phase is the local lesion at the bite, which starts as a papulovesicle and develops into a black eschar in 3 to 5 days. Fever and consti-tutional symptoms appear as the organism disseminates. The second phase of the disease is a diffuse rash similar to that of RMSF distributed randomly in the body, which, like the local lesion, becomes papulovesicular and develops into eschars. Rickettsialpox is self-limiting after 1 week, and no deaths have been reported. Tetracycline therapy shortens the course to 1 to 2 days.
TYPHUS GROUP
Epidemic Louse-Borne Typhus Fever
Primary louse-borne typhus fever is caused by Rickettsia prowazekii, which is transmitted to humans by the body louse. Historically, it has appeared during times of misery (war, famine) that create conditions favorable to human body lice (crowding, infrequent bathing). Although foci of endemic typhus are thought to persist in parts of Africa and Latin America, the number of reported cases has declined in recent decades. Most come from a single country, Ethiopia, which has had more than its share of social upheaval. Epidemic typhus has not been seen in the United States for more than half a century. R. prowazekii has been recovered from flying squirrels and their ectoparasites in thesoutheastern United States, and a few human cases of sylvatic typhus have occurred in these areas.
The chain of epidemic typhus infection starts with R. prowazekii circulating in a pa-tient’s blood during an acute febrile infection. The human body louse becomes infected during one of its frequent blood meals, and after 5 to 10 days of incubation, large num-bers of rickettsiae appear in its feces. As the louse defecates while it feeds, the organisms can be rubbed into the louse bite wounds when the host scratches the site. Dried louse fe-ces are also infectious through the mucous membranes of the eye or respiratory tract. The louse dies of its infection in 1 to 3 weeks, and the rickettsiae are not transmitted transo-varially.
Fever, headache, and rash begin 1 to 2 weeks after the bite. A maculopapular rash ap-pears first on the trunk and then spreads centrifugally to the extremities, a pattern oppo-site to that of RMSF. Headache, malaise, and myalgia are prominent components of the illness. Complications include myocarditis and central nervous system dysfunction. In untreated disease, the fatality rate increases with age from 10% to as high as 60%. Ther-apy with tetracycline or chloramphenicol is effective. Louse control is the best means of prevention and is particularly important in controlling epidemics. No effective vaccine is available.
Recrudescent Typhus
Recrudescent typhus (Brill’s disease) is a relapse of louse-borne typhus appearing 10 to 40 years after the primary attack. Factors triggering the relapse are unknown, but may in-volve fading immunity to rickettsiae that have remained dormant in reticuloendothelial cells. Recrudescent typhus is usually milder than the primary infection and is less often fatal, presumably because of partial immunity.
Endemic Typhus
Endemic or murine typhus is caused by Rickettsia typhi and transmitted to humans by the rat flea (Xenopsylla cheopis). Human illness is incidental to the natural transmission of the disease among urban rodents, which serve as the reservoir. Only 30 to 60 cases of murine typhus are reported in the United States each year. Half of these typically occur along the Gulf Coast of Texas.
The pathogenesis is similar to that of louse-borne typhus but the history includes exposure to rats, rat fleas, or both. The flea defecates when it takes a blood meal, and the infected feces gain access through the bite wound. After an incubation period of 1 to 2 weeks, illness begins with headache, myalgia, and fever. The rash is maculopapular, not petechial; it starts on the trunk and then spreads to the extremities in a manner similar to typhus. Because of antigens shared by R. typhi and R. prowazekii, serologic tests may not separate the two diseases. In the untreated patient, fever may last 12 to 14 days. With tetracycline or chloramphenicol therapy, the course is reduced to 2 to 3 days. Mortality and complications are rare, even if the disease is untreated.
Scrub Typhus
Scrub typhus is found in the southwest Pacific, Southeast Asia, and Japan. The causative organism is Orientia tsutsugamushi, a rickettsial organism. Mites that infest rodents are the reservoir and vectors, transmitting the rickettsiae to their own progeny via infected ova. Humans pick up the mites as they pass by low trees or brush. The mite larvae (chig-gers) deposit rickettsiae as they feed.
The typical initial lesion, a necrotic eschar at the site of the bite on the extremities, develops in only 50 to 80% of cases. Fever increases slowly over the first week, some-times reaching 40.5°C. Headache, rash, and generalized lymphadenopathy follow later.
The maculopapular rash, which appears after about 5 days, is more evanescent than that seen with louse-borne or murine typhus. Hepatosplenomegaly and conjunctivitis may also appear. Specific diagnosis requires demonstration of a serologic response using the IFA The prognosis is good with chloramphenicol or tetracycline therapy, but the mortal- ity of untreated patients is as high as 30%.
Related Topics