Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Insulin
Regular and Rapid Acting Soluble Formulations - Pharmacology and Formulations of Insulin
Regular and Rapid-Acting Soluble Formulations
Initial soluble insulin formulations were prepared under acidic
conditions and were chemically unstable. In these early formulations,
considerable deamidation was identified at AsnA21 and significant potency loss was
observed during prolonged storage under acidic conditions. Efforts to improve
the chemical stability of these soluble formulations led to the development of
neutral, zinc-stabilized solutions.
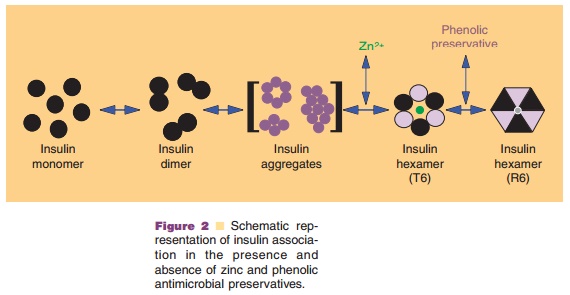
The insulin in these neutral, regular formula-tions is chemically
stabilized by the addition of zinc (~0.4%
relative to the insulin concentration) and phenolic preservatives. As mentioned
above, the addition of zinc leads to the formation of discrete hexameric
structures (containing 2 Zn atoms per hexamer) that can bind six molecules of
phenolic preservatives, e.g., m-cresol (Fig. 2). The binding of these
excipients increases the stability of insulin by inducing the formation of a
specific hexameric conformation (R ), in which the B1 to B8 region of each
monomer is in an α-helical conformation. This in turn decreases the
availabil-ity of residues involved in deamidation and high-molecular-weight
polymer formation (Brange et al., 1992a,b).
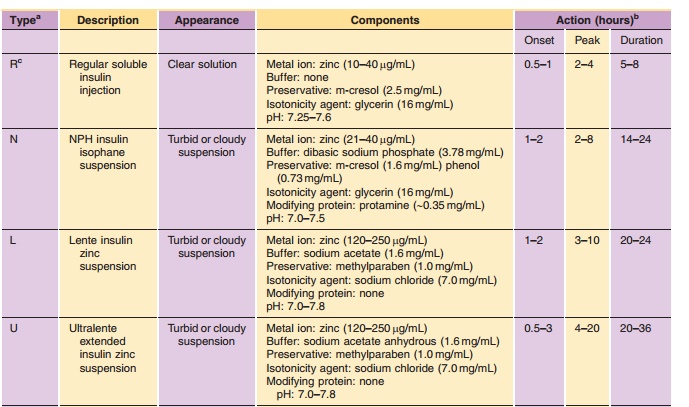
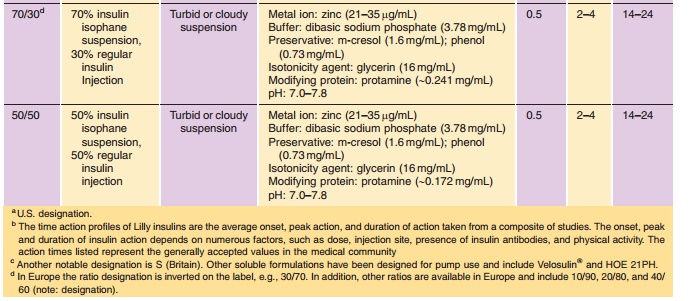
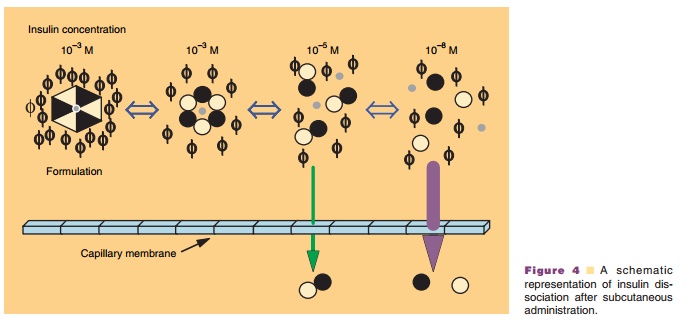
The pharmacodynamic profile of this soluble formulation is listed in Table 2. The neutral, regular formulations show peak insulin activity between 2 and 4 hr with a maximum duration of 5 to 8 hr. As with other formulations, the variations in time-action can be attributed to factors such as dose, site of injection, temperature, and the patient’s physical activity. Despite the soluble state of insulin in these formulations, a delay in activity is still observed. This delay has been attributed to the time required for the hexamer to dissociate into the dimeric and/or monomeric substituents prior to absorption from the interstitium. This dissociation requires the diffu-sion of the preservative and insulin from the site of injection, effectively diluting the protein and shifting the equilibrium from hexamers to dimers and monomers (Fig. 4) (Brange et al., 1990). Recent studies exploring the relationship of molecular weight and cumulative dose recovery of various compounds in the popliteal lymph following sub-cutaneous injection suggest that lymphatic transport may account for approximately 20% of the absorption of insulin from the interstitium (Supersaxo et al., 1990; Porter and Charman, 2000). The remaining balance of insulin is predominately absorbed through capillary diffusion.
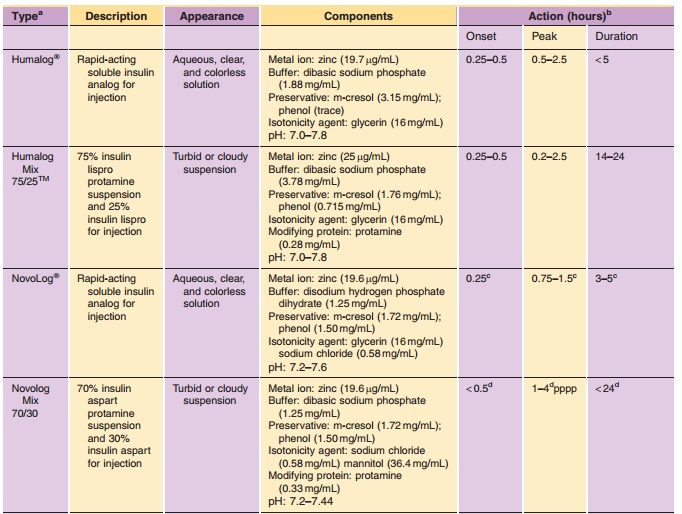
Monomeric insulin analogs were designed to achieve a more natural
response to prandial glucose-level increases while providing convenience to
thepatient. The pharmacodynamic profiles of these soluble formulations are
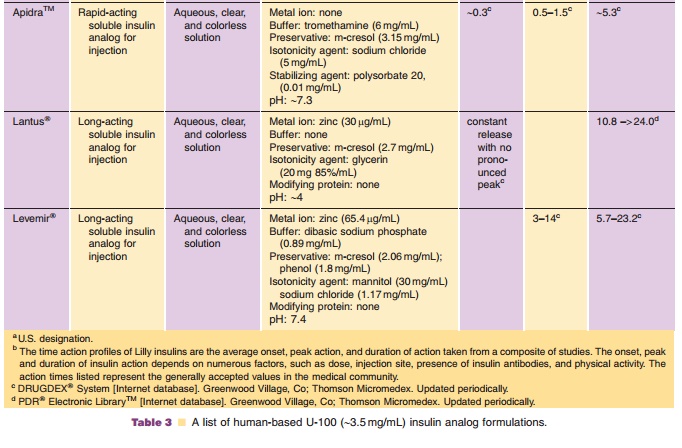
listed in Table 3. The development of monomeric analogs of insulin for the
treatment of insulin-dependent diabetes mellitus has focused on shifting the
self-association properties of insulin to favor the monomeric species and
consequently minimizing the delay in time-action
(Brange et al., 1988, 1990; Brems et al., 1992). One such monomeric
analog, LysB28ProB29-human insulin (Humalog or Liprolog ; insulin lispro; Eli Lilly &
Co., Lilly Corporate Center, Indianapolis, IN) has been developed and does have
a more rapid time-action profile, with a peak activity of approximately 1 hr
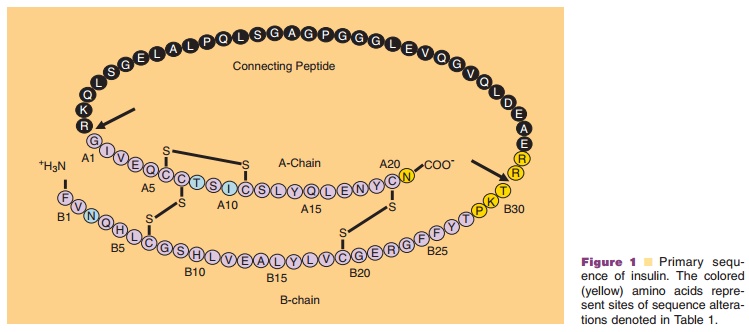
(Howey et al., 1994). The sequence inversion at positions B28 and B29 yields an
analog with reduced self-association behavior compared to human insulin (Fig.
1; Table 1); however, insulin lispro can bestabilized in a
preservative-dependent hexameric complex that provides the necessary chemical
and physical stability required by insulin preparations. Despite the hexameric
complexation of this analog, insulin lispro retains its rapid time-action.
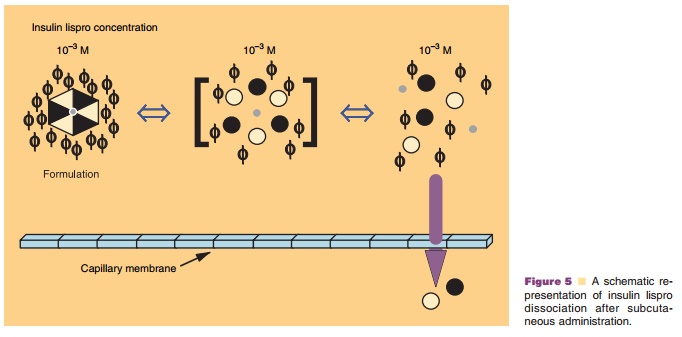
Based on the crystal structure of the insulin lispro hexameric complex, Ciszak
et al. (1995) have hypothesized that the reduced dimerization properties of the
analog, coupled with the preservative dependence, yields a hexameric complex
that readily dissociates into
It is important to highlight that the properties engineered into Humalog
not only provide the patient with a more convenient therapy, but also improve
control of postprandial hyperglycemia and reduce the frequency of severe
hypoglycemic events (Anderson et al., 1997; Holleman et al., 1997).
Since the introduction of insulin lispro, two additional rapid-acting
insulin analogs have been introduced to the market. The amino acid
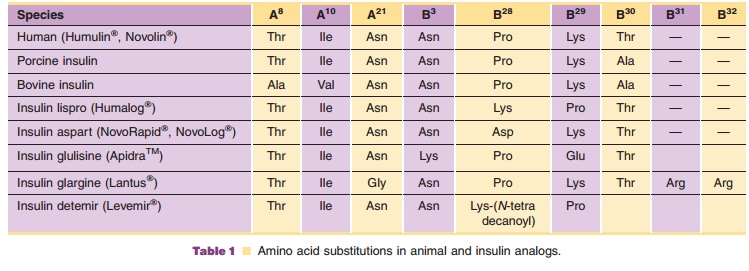
modifications made to the human insulin sequence to produce these analogs are
depicted in Table 1. Like insulin lispro, both analogs are supplied as neutral
pH solutions containing phenolic preservative. The design strategy for
AspB28-human insulin (NovoRapid or NovoLog; insulin aspart; Novo Nordisk A/S,
Corporate Headquarters, Novo Alle´, Bagsvaerd, Denmark) (Brange et al.,
1988, 1990) involves the replacement of ProB28 with a negatively charged aspartic acid residue. Like LysB28ProB29-human insulin, AspB28-human insulin has a more rapid
time-action following subcutaneous injection (Heinemann et al., 1997). This
rapid action is achieved through a reduction in the self-association behavior
compared to human insulin (Brange et al., 1990; Whittingham et al., 1998). The
other rapid-acting analog, LysB3-GluB29-human insulin (ApidraTM, insulin glulisine; Sanofi-Aventis), involves a substitution of the
lysine residue at position 29 of the B-chain with a negatively charged glutamic
acid. Additionally, this analog replaces the AsnB3 with a positively charged
lysine. Scientific reports describing the impact of these changes on the
molecular properties of this analog are lacking. However, the glutamic acid
substitution occurs at a position known to be involved in dimer formation
(Brange et al., 1990) and may result in disruption of key interactions at the
monomer– monomer interface. The Asn residue at position 3 of the B-chain plays
no direct role in insulin self-association (Brange et al., 1990), but it is flanked
by two amino acids involved in the assembly of the Zn2þ insulin hexamer. Despite the
limited physicochemical information on insulin glulisine, studies conducted in
persons with either Type 1 or Type 2 diabetes (Dailey et al., 2004; Dreyer et
al., 2005) confirm the analog displays similar pharmacological properties as
insulin lispro. Interestingly, insulin glulisine is not formulated in the
presence of zinc as are the other rapid-acting analogs. Instead, insulin
glulisine is formulated in the presence of a stabilizing agent (polysorbate 20)
(Table 3). The surfactant in the formulation presumably minimizes higher order
association and contributes to more rapid adsorption of the monomeric species.
In addition to the aforementioned rapid-acting formulations,
manufacturers have designed soluble formulations for use in external or
implanted infusion pumps. In most respects, these formulations are very similar
to Regular insulin (i.e., hexameric association state, preservative, and zinc);
however, buffer and/or surfactants may be included in these formulations to
minimize the physical aggregation of insulin that can lead to clogging of the
infusion sets. In early pump systems, gas-permeable infusion tubing was used
with the external pumps. Consequently a buffer was added to the formulation in
order to minimize pH changes due to dissolved carbon dioxide. Infusion tubing
composed of materials having greater resis-tance to carbon dioxide diffusion is
currently being used and the potential for pH-induced precipitation of insulin
is greatly reduced. All three of the commercially available rapid-acting
insulin analogs are approved for use in external infusion pumps.
Related Topics