Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Insulin
Long Acting Insulin Formulations - Pharmacology and Formulations of Insulin
Long-Acting Insulin Formulations
The normal human pancreas secretes approximately 1 unit of insulin
(0.035 mg) per hour to maintain basal glycemic control. Adequate basal insulin
levels are a critical component of diabetes therapy because they regulate
hepatic glucose output, which is essential for energy production by the brain.
Consequently, long-acting insulin formulation must provide a very different
pharmacokinetic profile than “meal-time” insulin formulation.
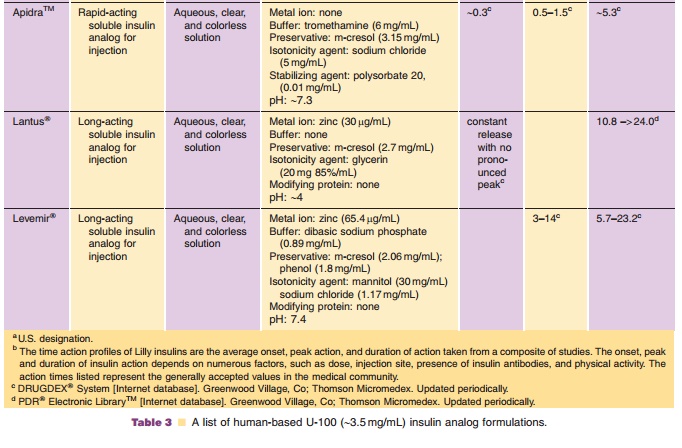
There are three long-acting insulin preparations currently commercially
available: Ultralente, which was developed in the 1950s and two insulin
analogs, Lantus (insulin glargine) and Levemir (insulin detemir), which have
been recently approved (Table 1; Fig. 1). Ultralente and Lantus derive
theirprotracted time-action profiles from the slow and relatively constant
dissolution of solid particles in the subcutaneous tissue. This slow
dissolution precedes the dissociation of insulin into absorbable units, and
thus the rate of absorption (units per hour) into the bloodstream is
significantly decreased in comparison to that of a solution (mealtime)
formulation. Levemir, on the other hand, achieves its protracted effect by a
combination of structural interactions and physiolo-gical binding events
(Havelund et al., 2004).
Ultralente is analogous to NPH insulin in that they are both formulated
as crystalline insulin suspensions. However, the preparations differ in several
key aspects. For one, under microscopic examination, the larger rhombohedral
Ultralente microcrystals are notably different than the much smaller rod-shaped
NPH microcrystals. This differ-ence originates from the different
crystallization conditions employed to prepare these formulations as well as
the excipients used. Ultralente contains no protamine and is crystallized at pH
5.5 in the presence of zinc, NaCl, and acetate buffer. The subsequent
formulation process involves adjustment of the pH to a final value of 7.4, with
the addition of excess zinc and methylparaben as an antimicrobial agent
(pre-servative). The different formulation excipients used for Ultralente in
comparison to NPH are a reflection of the different way in which the insulin molecules
are complexed into the respective crystal lattices. NPH crystals are believed
to be composed of zinc insulin hexamers stabilized as a complex with protamine
and preservative molecules (Balschmidt et al., 1991), whereas, Ultralente
crystals incorporate zinc insulin hexamers only (Brange, 1987a; Yip et al.,
1998). A consequence of this composition is that methylpar-aben must be
utilized as the preservative for Ultralente formulations because, unlike phenol
and m-cresol, it does not interact with and destabilize the Ultralente crystal
lattice.
As with all suspension products, Ultralente insulin should be uniformly
resuspended prior to withdrawal of the dose from the vial to ensure accurate
dosage. Ultralente has an onset of action of 0.5 to 3 hr, peak activity between
4 and 20 hr, and duration of action from 20 to 36 hr (Table 2). Similar to
other insulin formulations, the variations in time-action are due to factors
such as dose, site of injection, temperature, and the patient’s physical
activity. Ultralente may be mixed with Regular insulin and Humalog, although
its use in mixtures is constrained to extemporaneous mixing with immediate use
for the reasons outlined for Lente. Much like the Lente insulin formulation use
of Ultralente is declining
and its commercial manufacturing may soon cease. Insulin glargine
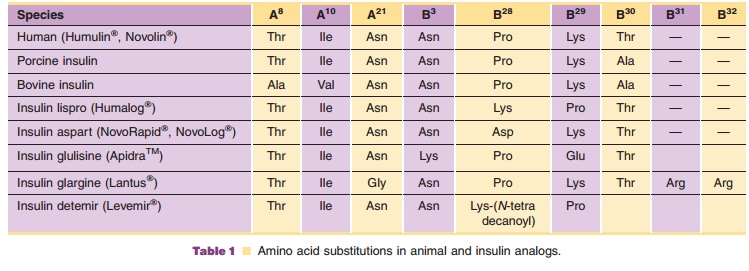
(Lantus; GlyA21, ArgB31, ArgB32
human insulin; Sanofi-Aventis), is a long-acting insulinanalog, whose
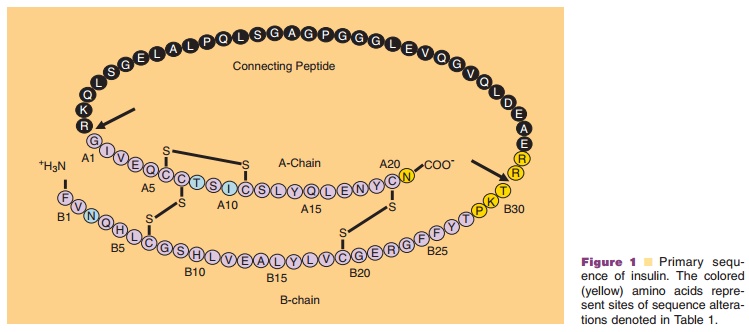
amino acid sequence modifications are highlighted in Table 1 and Figure 1. This
analog differs from human insulin in that the amino acid asparagine is replaced
with glycine at position A21 and two arginine residues have been added to the
C-terminus of the B-chain. The impact of the additional arginine residues is to
shift the pI from a pH of 5.4 to 6.7, thereby producing an insulin analog that
is soluble at acidic pH values, but is less soluble at the neutral pH of
subcutaneous tissue. Lantus is a solution formulation prepared under acidic
conditions, pH 4.0. The intro-duction of glycine at position A21 yields a
protein with acceptable chemical stability under acidic formulation conditions,
since the native asparagine is susceptible to acid-mediated degradation and
reduced potency. Thus, the changes to the molecular sequence of insulin have
been made to improve chemical stability and to modulate absorption from the
subcutaneous tissue, resulting in an analog that has approximately the same
potency as human insulin. The Lantus formulation is a clear solution that
incorporates zinc and m-cresol (preservative) at a pH value of 4. Consequently,
Lantus does not need to be resuspended prior to dosing. Immediately following
injection into the subcutaneous tissue, the insulin glargine precipitates due
to the pH change, forming a slowly dissolving precipitate. This results in a
relatively constant rate of absorption over 10.8 to 24 hr with no pronounced
peak (Table 3). This profile allows once-daily dosing as a patient’s basal
insulin. As with all insulin prepara-tions, the time course of Lantus may vary
in different individuals or at different times in the same individual and the
rate of absorption is dependent on blood supply, temperature, and the patient’s
physical activ-ity. Lantus should not be diluted or mixed with any other
solution or insulin, as will be discussed below.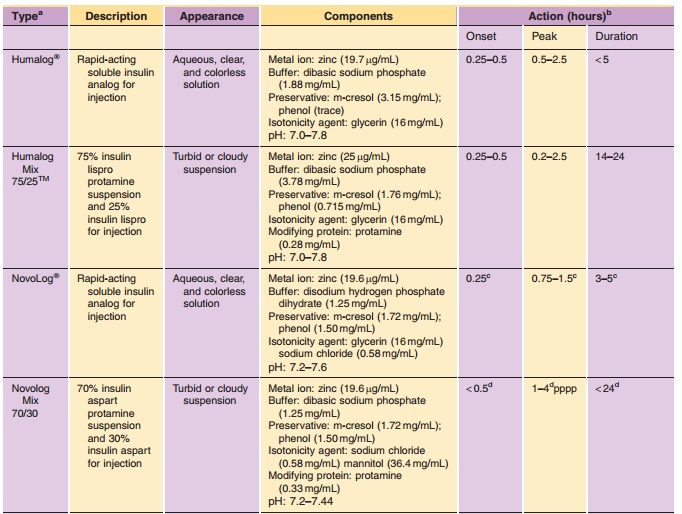
Insulin detemir (Levemir; LysB29(N-tetradeca-noyl)des(B30)human insulin; Novo-Nordisk A/S) utilizes
acylation of insulin with a fatty acid moiety as a means to achieve a
protracted pharmacological effect. As shown in Table 1 and Figure 1, the B30
threonine residue of human insulin is eliminated in insulin detemir and a
14-carbon, myristoyl fatty acid is covalently attached to the e-amino group of LysB29. The analog forms a zinc hexamer at neutral pH in a preserved solution.
Clinical studies have reported that insulin detemir displays lower
pharmacokinetic and pharmacodynamic variability than NPH (Hermansen et al.,
2001; Vague et al., 2003). An approximate description of the pharmacodynamic
profile of Levemir is listed in Table 3. This analog appears to display a
slower onset of action than NPH without a pronounced peak (Heinemann et al.,
1999). However, whether the duration of the protracted effect can truly be
considered sufficient enough to warrant classifica-tion of insulin detemir as a
long-acting insulinremains a subject of debate since published clinical studies
of this insulin analog are typically referenced to intermediate-acting NPH.
Binding of the tetradecanoyl-acylated insulin to albumin was originally
proposed as the underlying mechanism behind the observed prolonged effect for
the modified insulin analog; however, recent investi-gations on insulin detemir
have determined that the mechanism is more complex (Havelund et al., 2004). It
has been proposed that subcutaneous absorption is initially delayed as a result
of hexamer stability and dihexamerization. Such interactions between hex-amers
are a likely consequence of the symmetrical arrangement of fatty acid moieties
around the outside of the hexamers (Whittingham et al., 2004), as shown by
X-ray crystallographic studies. These associated forms further bind to albumin
within the injection site depot. Additional prolongation may result due to
albumin binding.
Related Topics