Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Insulin
Intermediate Acting Insulin Formulations - Pharmacology and Formulations of Insulin
Intermediate-Acting Insulin Formulations
There are two widely used types of intermediate-acting insulin
preparations: Neutral Protamine Hagedorn (NPH) and Lente. Both formulations
achieve extended time-action by necessitating the dissolution of a precipitated
and/or crystalline form of insulin. This dissolution is presumed to be the
rate-limiting step in the absorption of intermediate- and long-acting insulin.
Consequently, the time-action of the formulation is prolonged by further
delaying the dissociation of the hexamer into dimers and monomers.
NPH, named after its inventor H.C. Hagedorn (Hagedorn et al., 1936), is
a neutral crystalline suspension that is prepared by the cocrystallization of
insulin with protamine. Protamine consists of a closely related group of very
basic proteins that are isolated from fish sperm. Protamine is heterogeneous in
composition; however, four primary components have been identified and show a
high degree of sequence homology (Hoffmann et al., 1990). In general, protamine
is ~30 amino acids in length and has an amino acid composition that is
primarily composed of arginine, 65–70%. Using crystallization conditions
identified by Krayenbu¨hl and Rosenberg (1946), oblong tetragonal NPH insulin
crystals with volumes between 1 and 20 mm3 can be consistently prepared
from protamine and insulin (Deckert, 1980). These formulations, by design, have
very minimal levels of soluble insulin or protamine in solution. The condition
at which no measurable protamine or insulin exists in solution after
crystallization is referred to as the isophane point.
NPH has an onset of action from 1 to 2 hr, peak activity from 2 to 8 hr,
and duration of activity from 14 to 24 hr (Table 2). As with other
formulations, the variations in time-action are due to factors such as dose,
site of injection, temperature, and the patient’s physical activity.
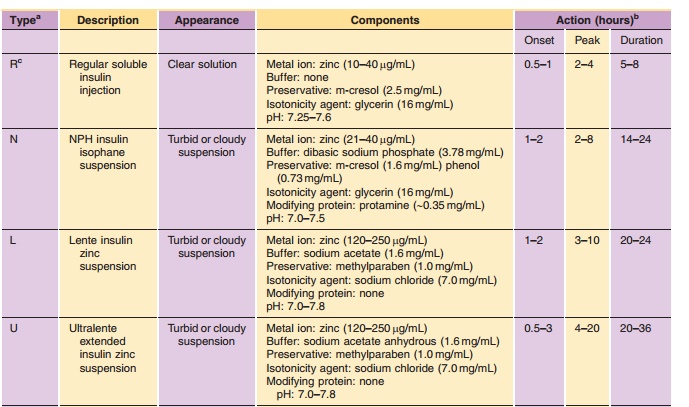
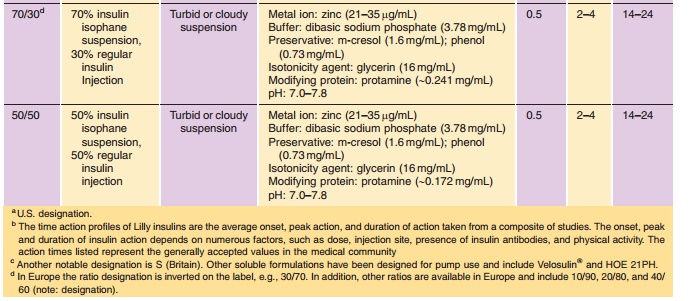
NPH can be readily mixed with Regular insulin either extemporaneously by the patient or as obtained from the manufacturer in a premixed formulation (Table 2). Premixed insulin, e.g., 70/30 or 50/50 NPH/Regular, has been shown to provide the patient with improved dose accuracy and consequently improved glycemic control (Bell et al., 1991). In these preparations, a portion of the soluble Regular insulin will reversibly adsorb to the surface of the NPH crystals through an electrostatically mediated interac-tion under formulation conditions (Dodd et al., 1995); however, this adsorption is reversible under physio-logical conditions and consequently has no clinical significance (Galloway et al., 1982; Hamaguchi et al., 1990; Davis et al., 1991). Due, in part, to the reversibility of the adsorption process, NPH/ Regular mixtures are uniquely stable and have a two-year shelf life.
The rapid-acting insulin analog, insulin lispro, can be extemporaneously
mixed with NPH; however, such mixtures must be injected immediately upon
preparation due to the potential for exchange between the soluble and
suspension components upon long-term storage. Exchange refers to the release of
human insulin from the NPH crystals into the solution phase and concomitant
loss of the analog into the crystalline phase. The presence of human insulin in
solution could diminish the rapid time-action effect of the analog. One way to
overcome the problem of exchange is to prepare mixtures containing the same
insulin species in both the suspension and the solution phases, analogous to
human insulin Regular/NPH preparations. However, this approach requires an
NPH-like preparation of the rapid-acting analog.
An NPH-like suspension of LysB28, ProB29-human insulin has been prepared and its physicochemical properties
relative to human insulin NPH have been described (DeFelippis et al., 1998). In
order to prepare the appropriate crystalline form of the analog, significant
modifications to the NPH crystallization procedure are required. The
differences between the crystallization conditions have been proposed to result
from the reduced self-association properties of LysB28, ProB29-human insulin.
Pharmacological studies reported for the LysB28, ProB29-human insulin NPH-like
suspension, com-monly referred to as neutral protamine lispro (NPL) (Janssen et
al., 1997; DeFelippis et al., 1998) indicate that the pharmacokinetic and
pharmacodynamic properties of this analog suspension are analogous to human
insulin NPH. The availability of NPL suspension allows for the preparation of
homoge-neous, biphasic mixture preparations containing intermediate-acting NPL
and rapid-acting solutions of LysB28, ProB29-human insulin that are not impacted by exchange between solution and
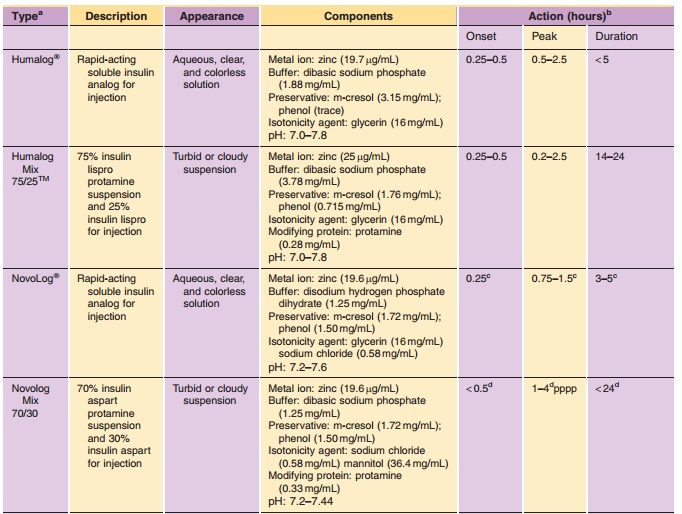
crystalline forms. As with LysB28, ProB29-human insulin, premixed formulations of the AspB28-human insulin have been prepared
in which rapid-acting soluble insulin aspart has been combined with a
protamine-retarded crystalline preparation of insulin aspart (Balschmidt,
1996). Clinical data on LysB28, ProB29-human insulin mix-tures and those composed of AspB28-insulin have been reported in
the literature (Weyer et al., 1997; Heise et al., 1998). The pharmacological
properties of the rapid-acting analogs are preserved in these stable mixtures
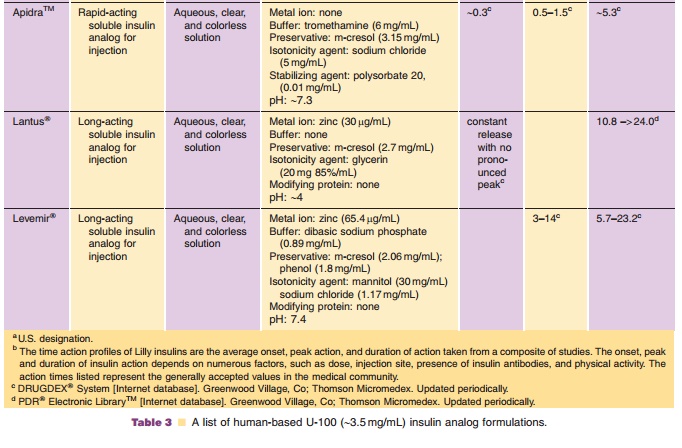
(Table 3). Premixed formulations of both rapid-acting analogs are now
commercially available in many countries.
Immunogenicity issues with protamine have been documented in a small
percentage of diabeticpatients (Kurtz et al., 1983; Nell and Thomas, 1988).
Individuals who show sensitivity to the protamine in NPH formulations are
routinely switched to Lente or Ultralente preparations to control their basal
glucose levels.
Lente insulin is a zinc insulin suspension that was designed for single
daily injection (Hallas-Møller et al., 1952). Lente insulin is a mixture of two
insoluble forms of insulin, 70% rhombohedral zinc insulin crystals (Ultralente
component) and 30% amorphous insulin particles (Semilente component). The pH of
the formulation is neutral and contains acetate and excess zinc. The surplus
zinc in the formulation presumably binds to weak-binding metal sites on the
insulin hexamer surface, reducing the solubility of the insulin, and thus
slowing the time-action of the insulin (Deckert, 1980). The crystal volume of
the Ultralente component is routinely between 200 and 1200 mm3 (Deckert, 1980).
Lente has an onset of action of 1–2 hr, peak activity from 3 to 10 hr,
and duration of action from 20 to 24 hr (Table 2). As with other formulations,
the variations in observed time-action are due to factors such as dose, site of
injection, temperature, and the patient’s physical activity.
The mixability of Lente with Regular insulin is restricted to
extemporaneous mixtures that are used immediately upon preparation (Deckert,
1980; Galloway et al., 1982). Prolonged storage of Lente/ Regular insulin
mixtures leads to a change in the course of effect due to precipitation of the
insulin from the Regular section (Deckert, 1980). The precipitation of Regular
insulin is presumably due to binding of the surplus zinc found in the Lente
formulation to weak-binding sites on the insulin hexamer. Use of Lente insulin
among persons with diabetes continues to decline and its continued commercial
viability is in doubt.
Related Topics