Chapter: Pharmaceutical Drug Analysis: Radioimmunoassay
Radioimmunoassay: Theory
THEORY
The basic underlying principle of radioimmunoassay utilizes the reaction between an antigen (hapten) and its specific antibody.
Small molecules (micromolecular) for instance : drugs that may serve as haptens
and can normally be made antigenic by coupling them chemically to a
macromolecular substance, such as : protein
polysaccharide, carbohydrate etc. The hapten is obtained from a non-antigenic
compound (micromolecule) e.g., morphine, cartelol etc., which is ultimately
conjugated*** convalently to a carrier**** macromolecule to render it
antigenic.
Animals normally develop antibodies***** to the injected immunogenic
substance as part of their natural immune response. The serum derived from
these animals is used as the antibody source and tested with reference to their
specificity, sensitivity or affinity at their titer level. By specificity, is meant the lowest concentration of a
compound which can be detected in undiluted body fluid. Generally, it is
referred to as the ‘‘detection limit’’
or the ‘‘cut off level’’.
Sensitivity defines the degree to which an
assay can distinguish one compound from another of the same nature and an immunoassay
is a function of the particular antibody molecules contained in the antiserum.
Specificity of the antiserum is a function of the particular antigen used to
immunize the animal. Affinity usually measures how strongly bound is the
antigen to the antibody. Titer
refers to the concentration level of, in the context of the usage, antibody
contained in the obtained serum.
Immunological reactions by virtue of their specificity
allow the discrete identification of single molecular entities in the presence
of many-fold higher concentrations of either multiple or chemically identical
molecular entities. However, it is pertinent to be noted here that both immunological and immunochemical techniques are capable of providing the much sought
after assay systems for pharmaceutical
substances present in complex mixtures without the necessity of undergoing
through the tedious and cumbersome process of prior extraction and purification
required frequently for their respective biological and chemical tests.
Interestingly, the radioimmunochemical
methods possess the additional advantages of offering exquisite sensitivity
as well as enhanced specificity*.
1. HAPTEN DETERMINANTS AND PURITY : THE KEY TO IMMUNOLOGICAL SPECIFICITY
It has since been recognized as a well established
phenomenon that is possible to hook-up a micromolecule (drug) to a
macromolecule (protein, polypeptide, polysaccharide) to render it antigenic,
inject the resulting conjugate into an immunologically competent animal and
subsequently harvest antibodies which includes those bound to the hapten
moiety. Nevertheless, the animal should be genetically a responder with regard
to the specific macromolecule carrier and even so to the micromolecule moiety
of the immunogenic conjugate. Apparently, it may appear as the most efficient
and easiest means to hook-up the micromolecule being made haptenic by any of
its available chemically reactive functional groups to the selected carrier
molecule.
But unfortunately, no matter how many competent animals
are immunized with such an immunogenic conjugate, the antisera thus generated
cannot contain a population in the total antibody immunoglobulin (IgG) pool
that will recognize the chemically reactive group used for coupling to the
carrier portion of the conjugate moiety. In case, only a small quantum of
antigenic determinants** exist in the hapten before conju-gation to
macromolecule the loss of even one functional group can turn out to be
critical.
2. IMPORTANCE OF ANTIGENIC DETERMINANTS
These are, namely :
(i) The
functional groups of the hapten should remain unblocked in the conjugate
molecule,
(ii) These chemical
functions are primarily responsible for metabolic activity ; besides, all
active functions of a small hapten should remain accessible in the hapten
carrier conjugate to obtain the most exquisitely specific antibody
immunoglobulin (IgG) population of which the immune system is capable,
(iii) The fewer
the active functions are available to serve as haptenic determination, the
lesser will be the specificity of the reaction in radioimmunoassay ; in other
words, the greater the number of antigenic determinants in a hapten molecule
the more specific shall be its reaction with its antibody.
Example : Blockade of a single hydroxyl
group of morphine in the preparation of morphine immunogen results in an
antiserum that is entirely unable to distinguish homologous morphine forms from
its corresponding surrogates with
unavailable hydroxyl(s)***. Further, the antiserum produced by immunization with such a morphonyl immunogen reacts
with codeine either equally or better than morphine.
(iv) All
chemically reactive functions of a pure derivative, not particularly those
which coincide with physiological activity, must remain undistorted and
accessible to avail themselves as immunological determinants.
3. ANALYSIS BY COMPETITIVE ANTIBODY BINDING OR ISOTOPICALLY LABELLED COMPOUNDS
Radioimmunoassay is nothing but a competitive binding
assay employing the principle of reversible binding of a labelled antigen to
its specific antibody ; and the ability of unlabelled antigen not only to
compete in the reaction but also to displace labelled antigen from antibody.
Nevertheless, the antibody and labelled antigen are always present as limiting
factors and the concentration of unlabelled antigen (present either as standard
solution or as sample under examination) is increased continually. It has been
observed that the percentage of antibody-bound labelled antigen declines
progressively as a consequence of saturation of the combining sites on the
antibody molecule.
The principle governing radioimmunoassay has been duly
illustrated in Figure 32.1.

An ideal behaviour has been assumed in Figure 32.1,
whereby most radioimmunoassay very closely approach this condition. In order to
fulfill the requirements of an ideal behaviour the following criteria must be
accomplished, namely :
(i) The
non-radioactive antigen (A) and radioactive antigen (A*) are indistinguishable
chemically i.e., both of them are
identical chemically,
(ii) The two
reactions ultimately go to completion i.e.,
the equilibrium constants of the binding of labelled and unlabelled antigen to
antibody are not only equal but also are so huge in number that they may be
regarded as infinite,
(iii) The
antigen and antibody usually react in the ratio 1 : 1, and
(iv) There are
no cross reactions observed in the medium i.e.,
the antibody being specific only for the single antigen indicated in the
reaction or being determined.
The main objective of RIA is to determine the
concentration ‘C’ of a non-radioactive antigen (unla-belled). Hence, in order
to conduct RIA-a standard curve first to be made where ‘C’, concentration of
non-labelled antigen in standard solution, is plotted as a function of
radioactivity. It is usually accomplished by saturating the antibody binding
sites with radioactive or labelled antigen, adding known concentration of the
non-radioactive (hapten) antigen, in standard solution, to the reaction mixture
for the unlabelled antigen from its binding site on the antibody. It is a
normal practice, to measure radioactivity with each known unlabelled antigen
added (concentration) which is plotted along the X-axis against the
radioactivity Y-axis. This is also known as the ‘close-response curve’.
If a radioactive-labelled form of a substrate (A*) is
added to a plasma containing unlabelled-substrate
and a limited amount of its specific binding antibody
(P), then assuming a dynamic equilibrium exists between (A) and (P), (A*) shall
distribute itself evenly among the unlabelled substrate (A). If the binding
affinity between (A) and (P) is very high, virtually all the (A*) added will be
found until (P) is saturated and at equilibrium. Thus, we have :
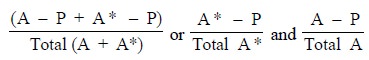
where, (A* – P) = Antibody
labelled antigen-complex, and
= (A – P) = antibody unlabelled antigen-complex.
At this juncture, if further (A) is added, it will also
compete for the same binding site so that (A* – P) shall be reduced. Still
further additions of (A) will cause the (A* – P) concentration to be reduced
further.
Under these prevailing circumstances the reduction in (A*
– P) complex concentration taking place may be predicted as follows :
Assuming that P (antibody) has 200 binding sites
available and at the initial stage only 20 molecules of (A) is present,
sufficient (A*) is added so as to saturate P i.e., 180 molecules of (A*). Therefore, virtually all are bound so
that :
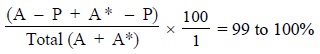
If, then 100 molecules of A are added, there is a total
of 300 molecules of (A* + A) competing for 200 binding sites on the antibody
(P). Now, when an equilibrium is established, the percentage bound is given by
the expression, :
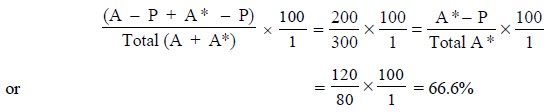
If a further 100 molecules of A are added at this stage,
the percentage bound shall become :
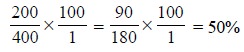
Thus, continuing with further additions of (A), each of
100 molecules at a time will ultimately give rise to two typical RIA-Standard
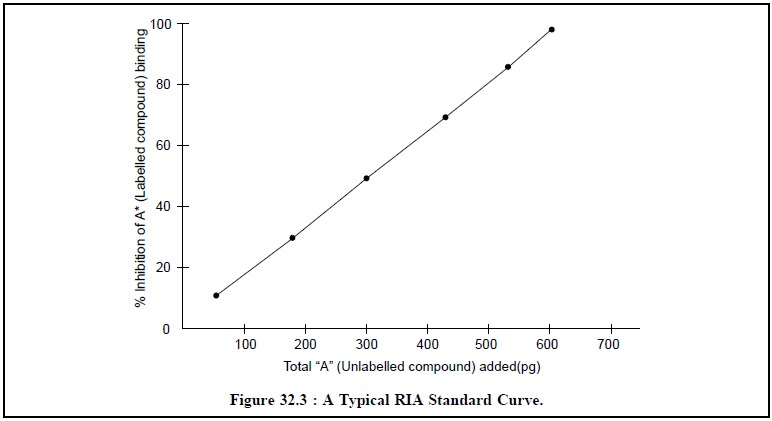
Curves as depicted in Figure 32.2 and Figure 32.3 respectively.
Form Figure 32.2, it is quite evident that the percentage
of radioactive compound bound A* decreases with the continual addition of
unlabelled compound A.
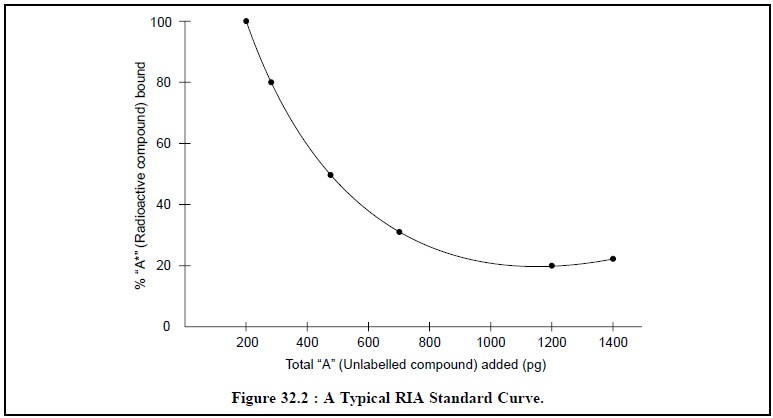
Figure 32.3, depicts the plotting of the percentage
inhibition of labelled compound binding A* against the continual addition of
unlabelled compound A thereby giving rise to a straight line.
The following important points may be observed :
(a) In place of
pure unlabelled A, a sample of plasma from which all the antibody P has been
removed duly, and which contains an unknown amount of A, is added to the same
system, it may be quantitated as per the respective observed fall in A* – P
complex concentration that it causes ultimately,
(b) It is
pertinent to mention here that the validity of radioimmunoassay procedure
solely depends upon the identical behaviour of standards as well as unknowns (i.e., unlabelled antigenic substance in
unknown sample being assayed). However, this particular condition may be tested
and verified by making multiple dilutions of an unknown sample and subsequently
determining whether the curve of competitive inhibition of binding is
superimposable on the standard curve employed for the respective assay. Failure
to fulfill this condition precludes a truly quantitative estimation+,
and
(c) A crude hormone
preparation is found to be satisfactory enough both for immunization and for
use as a standard, but for the purpose of comparison of values collected from
various laboratories, a generally available reference preparation must be used
as a standard solution.
Related Topics