Chapter: Essentials of Psychiatry: Mood Disorders: Premenstrual Dysphoric Disorder
Premenstrual Dysphoric Disorder: Treatment
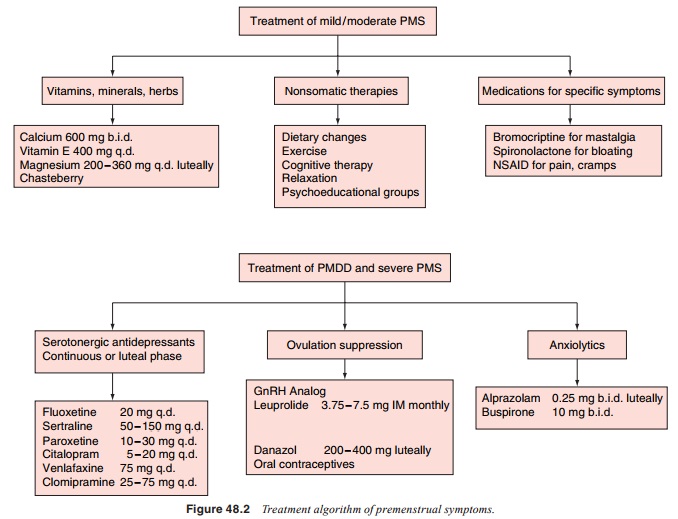
Treatment
Antidepressant Treatment
The treatment studies of SSRIs and venlafaxine in PMDD have suggested a
similar efficacy rate to treatment studies of SSRIs in major depressive
disorder, with 60 to 70% of women respond-ing to SSRIs compared with
approximately 30% of women re-sponding to placebo. In general, the effective
doses for all SSRIs are similar to the doses recommended for the treatment of
major depressive disorder (Figure 48.2) The efficacy of the continuous versus
intermittent dosing is equivalent.
A large RCT has reported that fluoxetine 20 mg/day dur-ing the luteal
phase only was superior to placebo in reducing premenstrual emotional and
physical symptoms in 252 women with PMDD (Cohen et al., 2002). There have not been reports of discontinuation
symptoms from these doses of SSRIs when abruptly stopped from the first day of
menses. There are no pub-lished studies to date of the efficacy of “symptom
onset” dosing of SSRIs, that is, administering SSRIs the postovulatory day that
premenstrual symptoms appear until menses. The efficacy of intermittent dosing,
as well as the findings from most SSRI tri-als that efficacy is achieved by the
first treatment cycle, has sug-gested a more rapid and different mechanism of
action of SSRIs in PMDD compared with its effect in major depressive disorder,
which typically takes 2 to 6 weeks. As discussed above, it has been
hypothesized that the rapid improvement of premenstrual symptoms by SSRIs may
be due to an increase in allopregna-nolone levels. The selective superiority of
serotonergic antide-pressants for PMDD is compatible with the postulated
serotonin dysfunction in PMDD.
Most SSRI trials have been 6 months or less in duration, so
efficacy-based long-term treatment recommendations do not ex-ist. Clinically,
many women note the recurrence of premenstrual symptoms after SSRI
discontinuation and many clinicians treat women over a long period of time. As
reviewed, a few open stud-ies report maintenance of SSRI efficacy over a couple
of years (Yonkers, 1997). Studies are needed to identify whether or not some
women develop tolerance to the SSRI and need a higher dose over time and
whether or not some women stay in remission for a period of time following SSRI
discontinuation.
Ovulation Suppression Treatments
Gonadotropin releasing hormone (GnRH) agonists suppress ovulation by downregulating GnRH receptors in the hypothalamus, leading to decreased follicle-stimulating hormone and luteinizing hormone release from the pituitary, resulting in decreased estrogen and progesterone levels. GnRH agonists are administered parenterally (e.g., subcutaneous monthly injections of goserelin, intramuscular monthly injections of leuprolide, daily intranasal buserelin) (see Figure 48.2). GnRH agonists lead to improvement in most emotional and physical premenstrual symptoms, with possible decreased efficacy for premenstrual dysphoria and severe premenstrual symptoms or for the exacerbation of chronic depression. After relief of PMS is achieved with a GnRH agonist, “add-back” hormone strategies have been investigated due to the undesirable medical consequences of the hypoestrogenic state resulting from prolonged anovulation. The addition of estrogen and progesterone to goserelin and leuprolide led to the reappearance of mood and anxiety symptoms. Since women with severe PMS and PMDD have an abnormal response to normal hormonal fluctuations, it is not surprising that women had the induction of mood and anxiety symptoms from the addition of gonadal steroids, reducing the benefit of the replacement strategy
Oral Contraceptives
Even
though oral contraceptives (OCs) are a commonly pre-scribed treatment for PMS,
there is minimal literature endorsing its efficacy. Anecdotally, women report
that oral contraceptives may benefit, worsen, or not affect their premenstrual
symptoms. The induction of dysphoria may be related to the type and dose of the
progestin component, the androgenic properties of the pro-gesterone, or to the
estrogen/progestin ratio (Kahn and Halbreich, 2001). A more recent RCT compared
an oral contraceptive to pla-cebo in 82 women with PMDD (Freeman et al., 2001).
Even though the OC containing ethinyl estradiol 30 µg and drospirenone 3 mg
improved most premenstrual symptoms, due in large part to a placebo response
rate of 40%, the OC was significantly more effi-cacious than placebo only in
decreasing food cravings, increased appetite and acne. Oral contraceptives have
been reported not to alter the response to SSRIs in women with PMDD.
Progesterone
The early assumption that PMS was due to a progesterone deficiency,
which has never been substantiated, led to luteal phase progesterone being one
of the earliest treatments of PMS in the literature. A recent systematic review
of published double-blind placebo-controlled randomized studies of luteal phase
progesterone (given as vaginal suppositories or oral micronized tablets) and
progestogens reported that there was no clinically meaningful difference
between all progesterone forms and placebo, although there was a small
statistically significant superiority of progesterone over placebo (Wyatt et al., 2001).
Other Medications
Alprazolam (administered during the luteal phase) has been re-ported to
be superior to placebo in most studies, and although it has a lower efficacy
rate than SSRIs, it is effective for premen-strual emotional symptoms.
Alprazolam should be tapered over the first few days of menses each cycle.
Buspirone at 25 mg/day during the luteal weeks has some efficacy.
Spironolactone has been reported to decrease premenstrual emotional and
physical symptoms. Bromocriptine has been reported to decrease premen-strual
breast.
Herbal Treatments and Dietary Supplementation
Most RCTs have shown little efficacy from herbal treatments and
therefore at this time no herbal treatment can be recommended. With respect to
dietary supplementation, calcium is reported to have a nearly 50% efficacy rate
for reducing the emotional and physical symptoms of the PMDD diagnostic
criteria, except for fatigue and insomnia, compared with 30% for placebo.
However, women with concurrent psychiatric illness were not clearly excluded,
and other treatments except for analgesics were allowed. The efficacy of
calcium was somewhat less in women who were also taking oral contraceptives
(Thys-Jacobs et al., 1998). The
results of this study were notable, and calcium deserves further study. Like herbal preparation, there is a
paucity of data to support the efficacy of vitamin supplementation. have a
nearly 50% efficacy rate for reducing the emotional and physical symptoms of
the PMDD diagnostic criteria, except for fatigue and insomnia, compared with
30% for placebo. However, women with concurrent psychiatric illness were not
clearly excluded, and other treatments except for analgesics were allowed. The
efficacy of calcium was somewhat less in women who were also taking oral
contraceptives (Thys-Jacobs et al.,
1998). The results of this study were notable, and calcium deserves further study. Like herbal preparation, there is a
paucity of data to support the efficacy of vitamin supplementation.
Lifestyle Modifications and Psychosocial Treatments
Many lifestyle modifications and psychosocial treatments have been
suggested for PMS. Lifestyle modifications are often sug-gested through
self-help materials or in an individual or group psychoeducation format. A
recent study reported that a weekly peer support and professional guidance
group for four sessions was superior to waitlist control in terms of reducing
premen-strual symptoms. The treatment consisted of diet and exercise regimens,
self-monitoring and other cognitive techniques and environment modification
(Taylor, 1999). Studies have not been conducted on individual lifestyle or
psychosocial treatments to identify which components are most efficacious.
Dietary recommendations include decreased caffeine, fre-quent snacks or
meals, reduction of refined sugar and artificial sweeteners, and increase in
complex carbohydrates. Premen-strual increased appetite and carbohydrate
craving increases the availability of tryptophan in the brain, leading to
increased serot-onin synthesis. There is little data at this time supporting
dietary interventions.
Exercise is likewise a frequently recommended treatment for PMS that has
yet to be tested in a sample of women with prospectively-confirmed PMS or PMDD.
As reviewed, negative effect and other premenstrual symptoms improve with
regular exercise in women in general. Cognitive therapy (CT) is reported to be
a promising treatment for PMS. There are limited studies in the use of light
therapy, massage therapy, reflexology, chiropractic manipulation, acupuncture
and biofeedback.
Related Topics