Chapter: Modern Pharmacology with Clinical Applications: Adrenomimetic Drugs
Pharmacodynamic Actions of Norepinephrine, Epinephrine, and Isoproterenol
PHARMACODYNAMIC
ACTIONS OF NOREPINEPHRINE, EPINEPHRINE, AND ISOPROTERENOL
Vascular Effects
The cardiovascular effects of
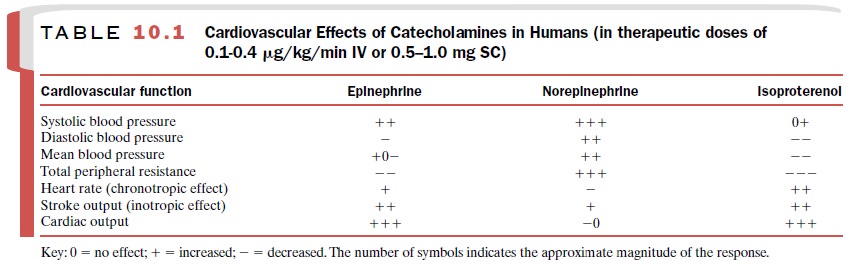
norepinephrine, epineph-rine, and isoproterenol are shown in Table 10.1.
Differences in the action of these three catecholamines on various vascular
beds are due both to the different affinities possessed by the catecholamines
for α- and β-adrenoceptors and to
differences in the relative dis-tribution of the receptors in a particular
vascular bed. The hemodynamic responses of the major vascular beds to these
amines are shown in Table 10.2.
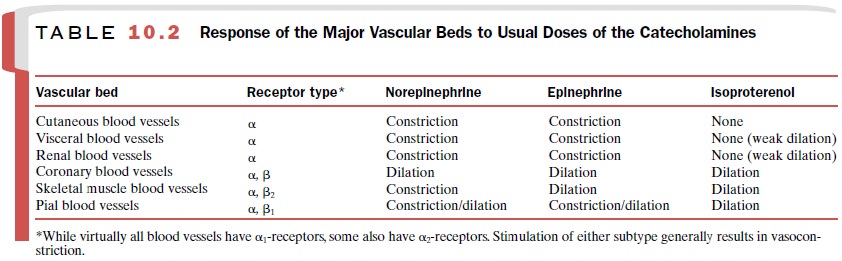
The blood vessels of the skin
and mucous mem-branes predominantly contain α-adrenoceptors. Both epinephrine and
norepinephrine produce a powerful constriction in these tissues, substantially
reducing blood flow through them. Isoproterenol, which is almost a pure β -adrenoceptor agonist, has
little effect on the vasculature of the skin and mucous membranes. The blood
vessels in visceral organs, including the kidneys, contain predominantly α-adrenoceptors, although some
β 2-adrenoceptors are also
present. Consequently, epi-nephrine and norepinephrine cause vasoconstriction and
reduced blood flow through the kidneys and other visceral organs. Isoproterenol
produces either no effect or weak vasodilation.
The blood vessels in skeletal muscle contain both α- and β 2-adrenoceptors. Norepinephrine constricts these blood vessels and reduces blood flow through an inter-action with α-adrenoceptors. Isoproterenol dilates the vessels in skeletal muscle and consequently increases blood flow through the tissue by interaction with the β 2-adrenoceptors.
Epinephrine has a more complex action on these blood vessels
because of its high affinity for both α- and β 2-adrenoceptors. Whether epinephrine produces
vasodilation or vasoconstriction in skeletal muscle depends on the dose
administered. Low doses of epinephrine will dilate the blood vessels; larger
doses will constrict them.
Although several factors can influence the flow of blood through
the coronary vessels, the most important of these is the local production of
vasodilator metabolites that results from stimulation-induced increased work by
the heart. α-Adrenoreceptors and β -adrenoceptors in the coronary vascular beds do not play
a major role in determining the vasodilator effects of the administra-tion of
epinephrine or norepinephrine.
Effects on the Intact Cardiovascular System
An increase in sympathetic
neuronal activity causes an increase in heart rate (positive chronotropic
effect, or tachycardia) and an increase in cardiac contractile force (positive
inotropic effect) such that the stroke output is increased. Cardiac output,
which is a function of rate and stroke output, is thus increased. A physiological in-crease in sympathetic
tone is almost always accompanied by a diminution of parasympathetic vagal
tone; this al-lows full expression of the effects of increased sympa-thetic
tone on the activity of the heart.
An increase in sympathetic
tone constricts blood vessels in most vascular beds and therefore causes a net
increase in total peripheral resistance. Increased sympa-thetic tone increases
neural release of norepinephrine and its interaction both with β-adrenoceptors on car-diac cells and with α-adrenoceptors on vascular
smooth muscle cells. As a consequence, the systolic and diastolic blood
pressures are elevated. It follows that the mean arterial blood pressure must
also be increased.
Norepinephrine
Norepinephrine, administered
to a normotensive adult either subcutaneously or by slow intravenous
in-jection, constricts most blood vessels. Venules as well as arterioles are
constricted. As a consequence, there is a net increase in the total peripheral resistance.
The effects of norepinephrine
on cardiac function are complex because of the dynamic interaction of the
direct effects of norepinephrine on the heart and the initiation of powerful
cardiac reflexes.
Important considerations are
as follows: (1) The di-rect effect of
norepinephrine on the heart is stimulatory. (2) The reflex initiated is
inhibitory, that is, opposite to the
direct effect. (3) The reflex varies with the level of sympathetic and
parasympathetic activity just before the initiation of the reflex. (4) The
distribution of sym-pathetic and parasympathetic nerves is not uniform in the
heart.
The net effect of
norepinephrine administration on heart rate and ventricular contractile force
therefore varies with the dose of norepinephrine, the physical ac-tivity of the
subject, any prior cardiovascular and baro-receptor pathology, and the presence
of other drugs that may alter reflexes.
In a normal resting subject
who is receiving no drugs, there is a moderate parasympathetic tone to the
heart, and sympathetic activity is relatively low. The ventricular muscle
receives little, if any, parasympathetic innervation. As the blood pressure
rises in response to norepinephrine,
the baroreceptor reflex is activated, parasympathetic impulses (which are inhibitory)
to the heart increase in frequency, and what little sympathetic outflow there
is may be reduced. Heart rate is slowed so much that the direct effect of
norepinephrine to in-crease the rate is masked and there is a net decrease in rate. Under the conditions
described, however, the im-pact of the reflex on the ventricles is very slight
because there is no parasympathetic innervation and the preex-isting level of
sympathetic activity is already low. A fur-ther decrease in sympathetic
activity therefore would have little further effect on contractility in this
subject. Thus, a decrease in heart rate and an increase in stroke volume will
occur, and cardiac output will change very little.
The reflex nature of the
bradycardia induced by parenterally administered norepinephrine can readily be
demonstrated by administration of atropine, a choli-noreceptor antagonist.
Atropine abolishes the com-pensatory vagal reflexes. Under conditions of vagal
blockade, the direct cardiac stimulatory effects of nor-epinephrine are
unmasked. There is marked tachycar-dia, an increase in stroke volume, and as a
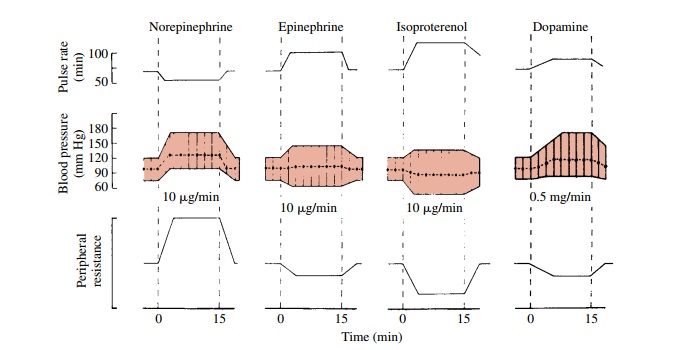
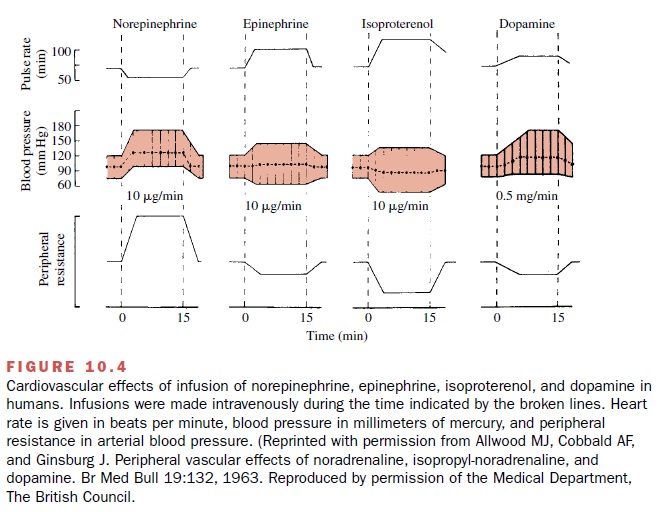
consequence, a marked increase in cardiac output (Fig. 10.4).
Epinephrine
A small dose of epinephrine
causes a fall in mean and diastolic pressure with little or no effect on
systolic pressure. This is due to the net decrease in total periph-eral
resistance that results from the predominance of vasodilation in the skeletal
muscle vascular bed. The in-travenous infusion or subcutaneous administration
of epinephrine in the range of doses used in humans gen-erally increases the
systolic pressure, but the diastolic pressure is decreased. Therefore, the mean
pressure may decrease, remain unchanged, or increase slightly, depending on the
balance between the rise in systolic and fall in diastolic blood pressures
(Fig. 10.4).
The cardiac effects of epinephrine are due to its ac-tion on β-adrenoceptors in the heart. The rate and con-tractile force of the heart are increased; consequently, cardiac output is markedly increased. Because total pe-ripheral resistance is decreased, the increase in cardiac output is largely responsible for the increase in systolic pressure. Since epinephrine causes little change in the mean arterial blood pressure, reflex slowing of the heart is usually not seen in humans.
Isoproterenol
Slow intravenous infusion of
therapeutic doses of isoproterenol in humans produces a marked decrease in
total peripheral resistance, owing to the predominance of vasodilation in
skeletal muscle vascular beds. As a consequence, diastolic and mean blood
pressures fall (Fig. 10.4). The depressor action of isoproterenol is more
pronounced than that of epinephrine because isopro-terenol causes no
vasoconstriction, whereas epinephrine does in some vascular beds. Systolic
blood pressure may remain unchanged or may increase. When an increase in
systolic blood pressure is seen, it is due to the marked in-crease in cardiac
output produced by isoproterenol.
Isoproterenol usually
increases the heart rate and stroke volume more than does epinephrine. This is
partly due to its ability to decrease mean blood pres-sure, which then
reflexively diminishes vagal activity, and partly to its action on the heart.
Effects on Vascular Smooth Muscle
Postjunctional α1-adrenoceptors are always
found in veins, arteries, and arterioles. Activation of these receptors results
in the entry of extracellular calcium through receptor-operated channels and in
the release of intra-cellularly stored calcium; this is brought about through
the participation of the inositol triphosphate second- messenger system. This
system plays an important role in the regulation of blood pressure and vascular
tone.
Vascular endothelium also
plays an important role in maintaining vascular tone.The endothelium can modulate
both vasodilation and vasoconstriction through its ability to locally
synthesize and release vasodilators such as nitric oxide, endothelium-derived
hyperpolarizing factor, and PGI2, and vasoconstrictors such as
endothelin, which in turn directly affect vascular smooth muscle activity.
Stimulation of α2-adrenoceptors located on the endothe-lial cells in certain
vascular beds (such as the coronary ar-tery) results in the release of nitric
oxide and vasodilation.
In any blood vessel, the
final integrated response to either neuronally released norepinephrine or to
circu-lating epinephrine probably depends on the relative participation of at
least four populations of α-adrenoceptors: postjunctional α 1- and α 2-adrenoceptors medi-ate constriction of
vascular smooth muscle, while pre-junctional and endothelial α 2-adrenoceptors mediate
vasodilation. An understanding of the vessel vascular response to adrenomimetic
drugs also must include the effects of drugs on adventitial innervation, smooth
mus-cle, and other vascular factors that may be present.
Effects on Nonvascular Smooth Muscle
In general, the responses to
administered catechol-amines are similar to those seen after sympathetic nerve
stimulation and depend on the type of adrenoceptor in the muscle.
Bronchial smooth muscle is relaxed by epinephrine and isoproterenol through their interaction with β2-adrenoceptors. Epinephrine
and isoproterenol are po-tent bronchodilators, while norepinephrine has a
rela-tively weak action in this regard .
Smooth muscle of the gastrointestinal tract is gener-ally relaxed by
catecholamines, but this may depend on the existing state of muscle tone.
Usually motility of the gut is reduced by catecholamines while the
gastroin-testinal sphincters are contracted. Catecholamines ap-pear to produce
relaxation of the gut through an action on α2-adrenoceptors on ganglionic cells. Activation
of these receptors reduces acetylcholine release from cholinergic neurons.
Catecholamines also may produce gastrointestinal relaxation through an action
on β2-adrenoceptors on smooth
muscle cells. Contraction of the sphincters occurs through an action on α1-adreno-ceptors. These
effects are quite transient in humans and therefore have no therapeutic value.
The radial (dilator) muscle of the iris contains α- adrenoceptors. Epinephrine
and norepinephrine cause dilation of the pupil (mydriasis) by contracting the dila-tor muscle.
Uterine muscle contains both α- and β-adrenocep-tors, which mediate contraction and relaxation,
respec-tively. The response of the human uterus to cate-cholamines is variable
and depends on the endocrine balance of the individual at the time of amine
adminis-tration . During the last stage of preg-nancy and during parturition,
epinephrine inhibits the uterine muscle, as does isoproterenol; norepinephrine
contracts the uterus.
The detrusor muscle (which contains β2-adrenocep-tors) in the body of the urinary
bladder is relaxed by epinephrine and isoproterenol. On the other hand, the
trigone and sphincter (which contain α1-receptors) are contracted by norepinephrine
and epinephrine; this ac-tion inhibits the voiding of urine.
Central Nervous System Effects
Epinephrine, in therapeutic
doses, mildly stimulates the CNS. The most noticeable features of this
stimulation are apprehension, restlessness, and increased respira-tion. In
therapeutic doses both isoproterenol and nor-epinephrine also have minor CNS
stimulant properties. Since these compounds do not easily cross the blood-brain
barrier, the mechanism of their stimulatory effects is not clear. It is likely
that the stimulating effects are primarily, if not entirely, due to actions in
the periphery that alter the neural input to the CNS.
Metabolic Effects
The catecholamines, primarily
epinephrine and isopro-terenol, exert a number of important effects on meta-
bolic processes. Most of these are mediated through an interaction with β-adrenoceptors.
Norepinephrine is usually effective only in large doses. Epinephrine and
isoproterenol in therapeutic doses increase oxygen con-sumption by 20 to 30%.
Endogenous epinephrine se-creted by the adrenal medulla in response to stress
such as exercise increases blood levels of glucose, lactic acid, and free fatty
acids.
Epinephrine, the most potent
stimulant of hepatic glycogenolysis, gives rise to glucose, which readily
en-ters the circulation; isoproterenol produces relatively weak hyperglycemia.
Administration of both α- and β-adrenoceptor blocking agents
is necessary for com-plete antagonism of glycogenolysis in this tissue.
Isoproterenol is the most
potent stimulant of skele-tal muscle glycogenolysis, followed by epinephrine
and norepinephrine. β2-Adrenoceptors mediate muscle glycogenolysis. Stimulation of
skeletal muscle glyco-genolysis will raise blood lactic acid levels rather than
blood glucose levels because skeletal muscle lacks the enzyme
glucose-6-phosphatase, which catalyzes the conversion of glucose-6-phosphate to
glucose.
The release of free fatty
acids from adipose tissue (lipolysis) is mediated through β3-adrenoceptors.
Iso-proterenol is the most potent agonist, followed by epi-nephrine and
norepinephrine.
Potassium Homeostasis
The catecholamines can play
an important role in the short-term regulation of plasma potassium levels.
Stimulation of hepatic α-adrenoceptors will result in the release of potassium from the
liver. In contrast, stimula-tion of β2-adrenoceptors, particularly in skeletal
muscle, will lead to the uptake of potassium into this tissue. The β2-adrenoceptors are linked to
the enzyme NA+ , K+ adenosine triphosphatase (ATPase).
Excessive stimula-tion of these β 2-adrenoceptors may produce hy-pokalemia, which
in turn can be a cause of cardiac ar-rhythmias.
Related Topics