Chapter: Clinical Anesthesiology: Perioperative & Critical Care Medicine: Fluid Management & Blood Component Therapy
Perioperative Fluid Therapy
Perioperative Fluid Therapy
Perioperative fluid therapy includes replacement of normal losses
(maintenance requirements), of pre-existing fluid deficits, and of surgical
wound losses including blood loss.
NORMAL MAINTENANCE REQUIREMENTS
In the absence of oral intake, fluid and electrolyte deficits can
rapidly develop as a result of contin-ued urine formation, gastrointestinal
secretions,
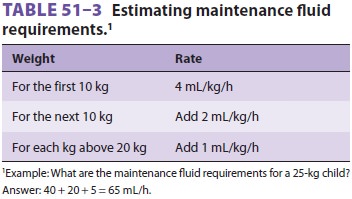
sweating, and insensible losses from the skin and lungs. Normal
maintenance requirements can be estimated from Table 51–3.
PREEXISTING DEFICITS
Patients presenting for surgery after an overnight fast without any
fluid intake will have a preexisting deficit proportionate to the duration of
the fast. The deficit can be estimated by multiplying the normal maintenance
rate by the length of the fast. For the average 70-kg person fasting for 8 h,
this amounts to (40 + 20 + 50) mL/h × 8 h, or 880 mL. In fact, the
real deficit is less as a result of renal conservation. (After all, how many of
us would feel the need to consume nearly 1L of fluid upon awakening after 8
hours of sleep?)
Abnormal fluid losses frequently contribute to preoperative deficits.
Preoperative bleeding, vomit-ing, diuresis, and diarrhea are often
contributory. Occult losses (really redistribution;) due to fluid sequestration
by traumatized or infected tis-sues or by ascites can also be substantial.
Increased insensible losses due to hyperventilation, fever, and sweating are
often overlooked.
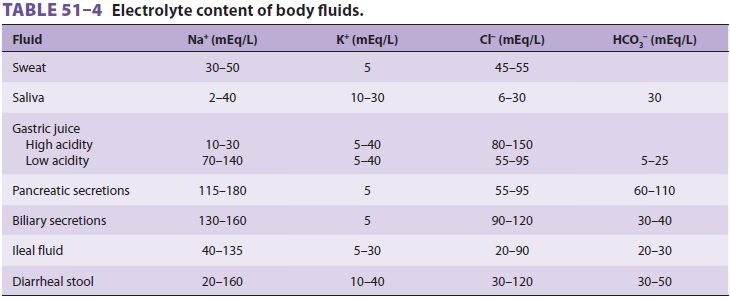
Ideally, deficits should be replaced preopera-tively in surgical
patients. The fluids used should be similar in composition to the fluids lost (Table
51–4).
SURGICAL FLUID LOSSES
Blood Loss
One of the most important, yet difficult, tasks of anesthesia personnel is to monitor and estimate blood loss. Although estimates are complicated by occult bleeding into the wound or under the surgical drapes, accuracy is important to guide fluid therapy and transfusion.
The most commonly used method for esti-mating
blood loss is measurement of blood in the surgical suction container and visual
estimation of the blood on surgical sponges (“4 by 4’s”) and lapa-rotomy pads
(“lap sponges”). A fully soaked 4 × 4 sponge is said to hold 10 mL of blood, whereas a soaked “lap” holds
100–150 mL. More accurate esti-mates are obtained if sponges and “laps” are
weighed before and after use, which is especially important during pediatric
procedures. Use of irrigating solu-tions complicates estimates, but their use
should be noted and an attempt made to compensate. Serial hematocrits or
hemoglobin concentrations reflect the ratio of blood cells to plasma, not
necessarily blood loss, and rapid fluid shifts and intravenous replacement
affect measurements.
Other Fluid Losses
Many surgical procedures are associated with oblig-atory losses of
fluids other than blood. Such losses are due mainly to evaporation and internal
redistri-bution of body fluids. Evaporative losses are most significant with
large wounds and are proportional to the surface area exposed and to the
duration of the surgical procedure.
Internal redistribution of fluids—often
called third-spacing—cancause massive
fluid shiftsand severe intravascular depletion. Everything related to
“third-space” fluid loss is controversial, including whether it actually exists
in patients other than those with peritonitis, burns, and similar situations
char-acterized by inflamed or infected tissue. Trauma-tized, inflamed, or
infected tissue can sequester large amounts of fluid in the interstitial space
and can translocate fluid across serosal surfaces (ascites) or into bowel
lumen. Shifting of intravascular fluid into the interstitial space is
especially important; protein-free fluid shift across an intact vascular
barrier into the interstitial space is exacerbated by hypervolemia, and
pathological alteration of the vascular barrier allows protein-rich fluid
shift.
INTRAOPERATIVE FLUID REPLACEMENT
Intraoperative fluid therapy should include supply-ing basic fluid
requirements and replacing residual preoperative deficits as well as
intraoperative losses (blood loss, fluid redistribution, and evaporation).
Selection of the type of intravenous solution depends on the surgical procedure
and the expected blood loss. For minor procedures involving minimal blood loss,
dilute maintenance solutions can be used. For all other procedures, lactated
Ringer’s solution or Plasmalyte is generally used even for maintenance
requirements.
Replacing Blood Loss
Ideally, blood loss should be replaced with crystal-loid or colloid
solutions to maintain intravascular volume (normovolemia) until the danger of
anemia outweighs the risks of transfusion. At that point, fur-ther blood loss
is replaced with transfusions of red blood cells to maintain hemoglobin
concentration (or hematocrit) at that level. There are no mandatory transfusion
triggers. The point where the benefits of transfusion outweigh its risks must
be considered on an individual basis.
Below a hemoglobin concentration of 7 g/dL,
the resting cardiac output increases to maintain a normal oxygen delivery. An
increased hemoglobin concentration may be appropriate for older and sicker
patients with cardiac or pulmonary disease, particularly when there is clinical
evidence (eg, a reduced mixed venous oxygen saturation and a per-sisting
tachycardia) that transfusion would be useful.
In settings other than massive trauma, most clinicians administer
lactated Ringer’s solution or Plasmalyte in approximately three to four times
the volume of the blood lost, or colloid in a 1:1 ratio, until the transfusion
point is reached. At that time, blood is replaced unit-for-unit as it is lost,
with reconstituted packed red blood cells.
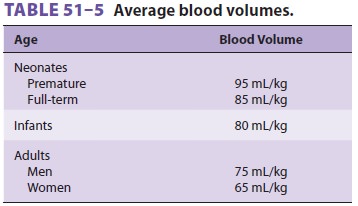
The transfusion point can be determined
pre-operatively from the hematocrit and by estimating blood
volume (Table 51–5). Patients with a normal hematocrit should generally betransfused only
after losses greater than 10–20% of their blood volume. The exact point is
based on the patient’s medical condition and the surgical procedure. The amount
of blood loss necessary for
the hematocrit to fall to 30% can be calculated as follows:
·
Estimate blood volume from Table
51–5.
·
Estimate the red blood cell volume
(RBCV) at the preoperative hematocrit (RBCVpreop).
·
Estimate RBCV at a hematocrit of 30%
·
(RBCV30%),
assuming normal blood volume is maintained.
·
Calculate the RBCV lost
when the hematocrit is 30%; RBCVlost= RBCVpreop – RBCV30%.
·
Allowable blood loss = RBCVlost× 3.
Example
An 85-kg woman has a preoperative hematocrit of 35%. How much blood loss
will decrease her hema-tocrit to 30%?
Estimated blood volume = 65 mL/kg × 85 kg = 5525 mL.
RBCV35%= 5525 × 35% = 1934 mL.
RBCV30%= 5525 × 30% = 1658 mL.
Red cell loss at 30% = 1934 − 1658 = 276 mL.
Allowable blood loss = 3 × 276 mL = 828 mL.
Th erefore, transfusion should be considered only when this patient’s
blood loss exceeds 800 mL. Increasingly, transfusions are not recommended until
the hematocrit decreases to 24% or lower (hemoglobin <8.0 g/dL), but it is necessary to take into account the rate of blood
loss and comorbid conditions (eg, cardiac disease, in which case trans-fusion
might be indicated if only 800 mL of blood is lost).
Clinical guidelines commonly used include:
(1) one unit of red blood cells will increase hemoglo-bin 1 g/dL and the
hematocrit 2–3% in adults; anda 10-mL/kg transfusion of red blood
cells will increase hemoglobin concentration by 3 g/dL and the hematocrit by
10%.
Replacing Redistributive & Evaporative Losses
Because redistributive and evaporative losses
are primarily related to wound size and the extent of surgical dissections and
manipulations, procedures can be classified according to the degree of tissue
trauma. These additional fluid losses can be replaced
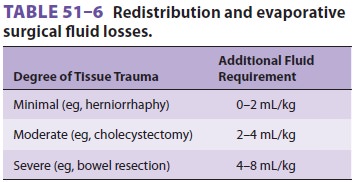
according to Table 51–6, based on whether tissue
trauma is minimal, moderate, or severe. These val-ues are only guidelines, and
actual needs vary con-siderably from patient to patient.
Related Topics