Chapter: Clinical Anesthesiology: Perioperative & Critical Care Medicine: Fluid Management & Blood Component Therapy
Evaluation of Intravascular Volume
Evaluation of Intravascular Volume
Clinical estimation of intravascular volume
must be relied upon because objective measurements of fluid compartment volumes
are not practical in the clini-cal environment. Intravascular volume can be
esti-mated using patient history, physical examination,and laboratory analysis,
often with the aid of sophisticated hemodynamic monitoring techniques.
Regardless of the method employed, serial evalua-tions are necessary to confirm
initial impressions and to guide fluid, electrolyte, and blood component
therapy. Multiple modalities should complement one another, because all
parameters are indirect, nonspecific measures of volume; reliance upon any one
parameter may lead to erroneous conclusions.
PATIENT HISTORY
The patient history is an important tool in preop-erative volume status
assessment. Important fac-tors include recent oral intake, persistent vomiting
or diarrhea, gastric suction, significant blood loss or wound drainage,
intravenous fluid and blood administration, and recent hemodialysis if the
patient has kidney failure.
PHYSICAL EXAMINATION
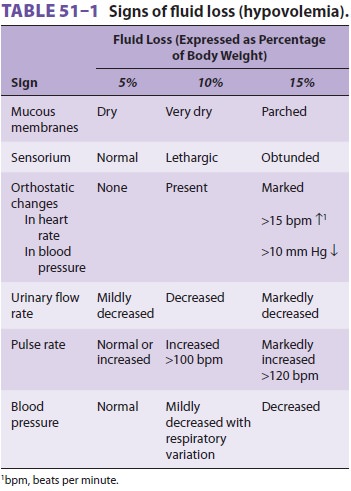
Indications of hypovolemia include abnormal skin turgor, dehydration of
mucous membranes, thready peripheral pulses, increased resting heart rate and
decreased blood pressure, orthostatic heart rate and blood pressure changes
from the supine to sitting or standing positions, and decreased urinary flow
rate (Table 51–1). Unfortunately, many medica-tions
administered during anesthesia, as well as the neuroendocrine stress response
to operative proce-dures, alter these signs and render them unreliable in the
immediate postoperative period. Intraopera-tively, the fullness of a peripheral
pulse, urinary flow rate, and indirect signs such as the response of blood
pressure to positive-pressure ventilation and to the vasodilating or negative
inotropic effects of anes-thetics, are most often used.
Pitting edema—presacral in the bedridden patient or pretibial in the
ambulatory patient—and increased urinary flow are signs of excess
extracel-lular water and likely hypervolemia in patients with normal cardiac,
hepatic, and renal function. Late signs of hypervolemia in settings such as
congestive heart failure may include tachycardia, elevated jugu-lar pulse
pressure, pulmonary crackles and rales, wheezing, cyanosis, and pink, frothy
pulmonary secretions.
LABORATORY EVALUATION
Several laboratory measurements may be used as surrogates of
intravascular volume and adequacy of tissue perfusion, including serial
hematocrits, arte-rial blood pH, urinary specific gravity or osmolality,
urinary sodium or chloride concentration, serum sodium, and the blood urea
nitrogen (BUN) to serum creatinine ratio. However, these measurements are only
indirect indices of intravascular volume, and they often cannot be relied upon
intraoperatively because they are affected by many perioperative fac-tors and
because laboratory results are often delayed. Laboratory signs of dehydration
may include rising hematocrit and hemoglobin, progressive metabolic acidosis
(including lactic acidosis), urinary specific gravity greater than 1.010,
urinary sodium less than 10 mEq/L, urinary osmolality greater than 450 mOsm/L,
hypernatremia, and BUN-to-creatinine ratio greater than 10:1. The hemoglobin
and hematocrit are usu-ally unchanged in patients with acute hypovolemiasecondary
to acute blood loss because there is insuf-ficient time for extravascular fluid
to shift into the intravascular space. Radiographic indicators of vol-ume
overload include increased pulmonary vascular and interstitial markings (Kerley
“B” lines) or diffuse alveolar infiltrates.
HEMODYNAMIC MEASUREMENTS
Central venous pressure (CVP) monitoring has been used in patients with
normal cardiac and pulmonary function when volume status is difficult to assess
by other means or when rapid or major alterations are expected. However, static
CVP readings do not provide an accurate or reliable indication of volume
status.
Pulmonary artery pressure monitoring has been
used in settings where central venous pressures do not correlate with the
clinical assessment or when the patient has primary or secondary right
ventricular dysfunction; the latter is usually due to pulmonary or left
ventricular disease, respectively. Pulmonary artery occlusion pressure (PAOP)
readings of less than 8 mm Hg indicate hypovolemia in the presence of
confirmatory clinical signs; however, values less than 15 mm Hg may be
associated with relative hypo-volemia in patients with poor ventricular
compliance. PAOP measurements greater than 18 mm Hg are elevated and generally
imply left ventricular volume overload. The normal relationship between PAOP
and left ventricular end-diastolic volume is altered by the presence of mitral
valve disease (particularly ste-nosis), severe aortic stenosis, or a left
atrial myxoma or thrombus, as well as by increased thoracic and pul-monary
airway pressures. All PAOP measurements should be obtained at end expiration
and interpreted in the context of the clinical setting. Finally, one should
recognize that multiple studies have failed to show that pulmo-nary artery
pressure monitoring leads to improved outcomes in critically ill patients, and
that echocar-diography provides a much more accurate and less invasive estimate
of cardiac filling and function.
Intravascular volume status is often difficult to
assess, and goal-directed hemodynamic and fluid therapy utilizing arterial
pulse contour analy-sis and estimation of stroke volume variation (eg,
LIDCOrapid, Vigileo FloTrak), esophageal Doppler, or transesophageal
echocardiography should be considered when accurate determination of
hemo-dynamic and fluid status is important. Stroke vol-ume variation (SVV) is
calculated as follows:
SVV = SVmax− SVmin/SVmean
The
maximum, minimum and mean SV are calculated for a set period of time by the
various measuring devices. During spontaneous ventilation the blood pressure
decreases on inspiration. During positive pressure ventilation the opposite
occurs. Normal SVV is less than 10–15% for patients on controlled ventilation.
Patients with greater degrees of SVV are likely to be responsive to fluid
therapy. In addition to providing a better assessment of the patient’s volume
and hemodynamic status than that obtained with CVP monitoring, these
modali-ties avoid the multiple risks associated with central venous and
pulmonary artery catheters.
Related Topics