Chapter: Clinical Anesthesiology: Perioperative & Critical Care Medicine: Fluid Management & Blood Component Therapy
Intravenous Fluids
Intravenous Fluids
Intravenous fluid therapy may consist of
infusions of crystalloids, colloids, or a combination of both. Crystalloid
solutions are aqueous solutions of ions (salts) with or without glucose,
whereas colloid solu-tions also contain high-molecular-weight substances such
as proteins or large glucose polymers. Colloid solutions help maintain plasma
colloid oncotic pres-sure and for the
most part remain intravascular, whereas crystalloid solutions rapidly
equilibrate with and distribute throughout the entire extracellular fluid
space.
Controversy exists regarding the use of
colloid versus crystalloid fluids for surgical patients. Propo-nents of
colloids justifiably argue that by maintaining plasma oncotic pressure,
colloids are more efficient (ie, a smaller volume of colloids than crystalloids
is required to produce the same effect) in restoring normal intravascular
volume and cardiac output. Crystalloid proponents, on the other hand, maintain
that the crystalloid solutions are equally effective when given in appropriate
amounts. Concerns that colloids may enhance the formation of pulmonary edema f
luid in patients with increased pulmonary capillary permeability appear to be
unfounded . Several generalizations can be made:
·
Crystalloids, when given in
sufficient amounts, are just as effective as colloids in restoring
intravascular volume.
·
Replacing an
intravascular volume deficit withcrystalloids generally requires three
to fourtimes the volume needed when using colloids.
·
Surgical patients may have
an extracellular fluid deficit that exceeds the intravascular deficit.
·
Severe intravascular
fluid deficits can be more rapidly corrected using colloid solutions.
·
The rapid administration of large
amounts of crystalloids (>4–5 L) is
more frequently associated with tissue edema.Some evidence suggests that marked
tissue edema can impair oxygen transport, tissue heal-ing, and return of bowel
function following major surgery.
CRYSTALLOID SOLUTIONS
Crystalloids are usually considered as the initial resuscitation fluid
in patients with hemorrhagic and septic shock, in burn patients, in patients
with head injury (to maintain cerebral perfusion pressure), and in patients
undergoing plasmapheresis and hepatic resection. Colloids may be included in
resuscitation efforts following initial administration of crystalloid solutions
depending upon anesthesia provider pref-erences and institutional protocols.
A wide variety of solutions is available (Table 51–2), and choice is according to
the type of
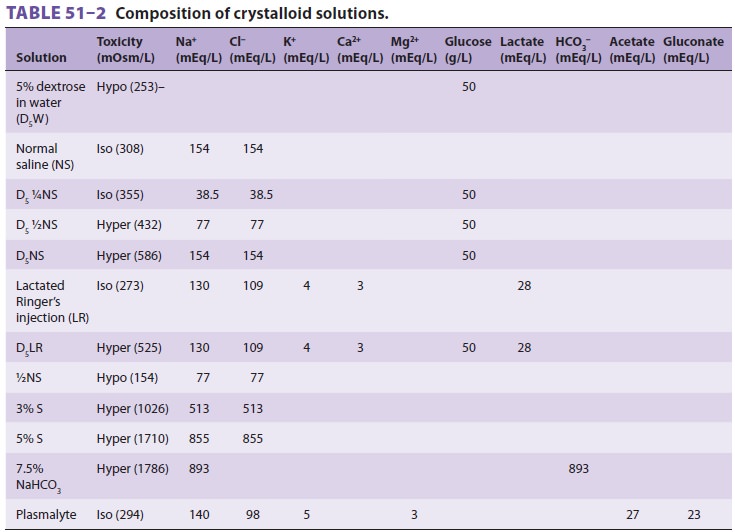
fluid loss being replaced. For losses
primarily involv-ing water, replacement is with hypotonic solutions, also
called maintenance-type solutions. If losses involve both water and
electrolytes, replacement is with isotonic electrolyte solutions, also called
replacement-type solutions. Glucose is provided in some solutions to maintain
tonicity, or prevent keto-sis and hypoglycemia due to fasting, or based on
tradition. Children are prone to developing hypo-glycemia (<50 mg/dL) following 4- to 8-h fasts.
Because most intraoperative fluid losses are iso-tonic, replacement-type
solutions are generally used. The most commonly used fluid is lactated Ringer’s
solution. Although it is slightly hypotonic, provid-ing approximately 100 mL of
free water per liter and tending to lower serum sodium, lactated Ringer’s
generally has the least effect on extracellular fluid composition and appears
to be the most physiologi-cal solution when large volumes are necessary. The
lactate in this solution is converted by the liver into bicarbonate. When given in largevolumes, nor-mal saline produces a
dilutional hyperchloremic acidosis because of its high sodium and chloride
content (154 mEq/L): plasma bicarbonate con-centration decreases as chloride
concentration increases. Normalsalineis thepreferred
solutionforhypochloremic metabolic alkalosis and for diluting packed red blood
cells prior to transfusion. Five per-cent dextrose in water (D5W) is used
for replacement of pure water deficits and as a maintenance fluid for patients
on sodium restriction. Hypertonic 3% saline is employed in therapy of severe
symptomatic hypo-natremia . Hypotonic solutions must be administered slowly to
avoid inducing hemolysis.
COLLOID SOLUTIONS
The osmotic activity of the high-molecular-weight
substances in colloids tends to maintain these solutions
intravascularly. Although the intravascu-lar half-life of a crystalloid
solution is20–30 min, most colloid solutions have
intravascu-lar half-lives between 3 and 6 h. The relatively greater cost and
occasional complications associated with colloids may limit their use.
Generally accepted indications for colloids include (1) f luid resuscita-tion
in patients with severe intravascular fluid deficits (eg, hemorrhagic shock)
prior to the arrival of blood for transfusion, and (2) fluid resuscitation in
the presence of severe hypoalbuminemia or con-ditions associated with large
protein losses such as burns. For burn patients, colloids are not included in
most initial resuscitation protocols (and we strongly recommend that burn
surgeons and anesthesia per-sonnel develop a resuscitation protocol and follow
it), but may be considered following initial resuscita-tion with more extensive
burn injuries during subse-quent operative procedures.
Many clinicians also use colloid solutions in
conjunction with crystalloids when f luid replace-ment needs exceed 3–4 L prior
to transfusion. It should be noted that colloid solutions are prepared in
normal saline (Cl − 145–154 mEq/L) and thus can also cause hyperchloremic metabolic
acidosis (see above). Some clinicians suggest that during anesthe-sia,
maintenance (and other) fluid requirements be provided with crystalloid
solutions and blood loss be replaced on a milliliter-per-milliliter basis with
colloid solutions (including blood products).
Several colloid solutions are generally available. All are derived from
either plasma proteins or syn-thetic glucose polymers and are supplied in
isotonic electrolyte solutions.
Blood-derived colloids include albumin (5% and 25% solutions) and plasma
protein fraction (5%). Both are heated to 60°C for at least 10 h to minimize the risk of transmitting hepatitis and
other viral diseases. Plasma protein fraction contains α-and β-globulins in addition to
albumin and has occasionally resulted in hypotensive reactions. These reactions
are allergic in nature and may involve acti-vators of prekallikrein.
Synthetic colloids include dextrose starches
and gelatins. Gelatins are associated with histamine-mediated allergic
reactions and are not available in the United States. Dextran is available as dextran (Macrodex) and dextran 40 (Rheomacrodex), which
have average molecular weights of 70,000 and 40,000, respectively. Although
dextran 70 is a better volume expander than dextran 40, the latter also
improves blood flow through the microcircu-lation, presumably by decreasing
blood viscosity, and is often administered to take advantage of these
rheological properties rather than to meet “fluid requirements.” Antiplatelet
effects are also described for dextrans. Infusions exceeding 20 mL/kg per day
can interfere with blood typing, may prolong bleed-ing time, and have been
associated with kidney fail-ure. Dextrans can also be antigenic, and both mild
and severe anaphylactoid and anaphylactic reactions are described. Dextran 1
(Promit) may be adminis-tered prior to dextran 40 or dextran 70 to prevent
severe anaphylactic reactions; it acts as a hapten and binds any circulating
dextran antibodies.
Hetastarch (hydroxyethyl starch) is available
in multiple formulations, which are designated by concentration, molecular
weight, degree of starch substitution (on a molar basis), and ratio of
hydrox-ylation between the C2 and the C6 positions. Thus in some countries a
wide variety of formulations are available with concentrations between 6% and
10%, molecular weights between 200 and 670, and degree of molar substitution
between 0.4 and 0.7. A greater ratio of C2 versus C6 substitution leads to
longer per-sistence in plasma. The starch molecules are derived from plants.
Smaller starch molecules are eliminated by the kidneys, whereas large molecules
must first be broken down by amylase. Hetastarch is highly effec-tive as a
plasma expander and is less expensive than albumin. Moreover, hetastarch is
nonantigenic, and anaphylactoid reactions are rare. Coagulation stud-ies and
bleeding times are generally not significantly affected following infusions of
older, higher molecu-lar weight formulations up to 1.0 L in adults. Newer,
lower molecular weight formulations can safely be given in larger volumes.
Related Topics