Chapter: Surgical Pathology Dissection : The Endocrine System
Pediatric Tumors : Surgical Pathology Dissection
Pediatric Tumors
General Comments
A number
of tumors are unique to children. These tumors frequently require special
processing to ensure that the diagnosis can be established and appropriate
treatment given. The Children’s On-cology Group (COG) creates, monitors, and
eval-uates therapeutic protocols for pediatric tumors. In the United States,
COG frequently requires that pathologic material be sent to review
patholo-gists to verify the diagnosis and to further classify the tumor.
Additional fresh and frozen tissue is often required for biologic studies,
which are performed at specialized reference laborato-ries. If this tissue is
not collected at the time of surgery, the patient may not be eligible for the
appropriate treatment protocol. Furthermore, as knowledge is accumulated and
protocols change, tissue requirements change. Therefore, patholo-gists who are
processing pediatric tumors need to work closely with their pediatric
oncologists to be aware of the current protocol requirements. Conversely, the
entire specimen cannot be submitted for biologic studies. Sufficient tissue
must be available to establish a histologic diag-nosis. It is vital that the
pathologist be respon-sible for the appropriate triage of this tissue. In many
cases, tissue also needs to be submitted for ancillary diagnostic studies (such
as electron microscopy and cytogenetic analysis). Despite these demands,
pediatric tumors can be pro-cessed easily if a series of steps is routinely
performed at the time of initial processing. Establishing a routine is
particularly important because many biopsies are performed during off-hours.
Overall Guidelines
1. Ensure
that all pediatric tumors are
promptly received in the fresh state. This may entail pro-cessing during
off-hours by on-call personnel.
2. Decide
how much of the specimen is needed for routine histology. This will depend on
the tumor type suspected and the size of the biopsy.
3. Submit
tissue for electron microscopy if appro-priate. It is good practice to put a
small piece of every pediatric tumor in glutaraldehyde. This can then be
embedded, and the decision of whether to section and process can be made at a
later time.
4. Place 1/2 to 1 cc
of minced tumor in Roswell Park Memorial Institute medium (RPMI) or equivalent
tissue culture medium and refrig-erate. This material can be submitted for
cyto-genetic analysis, flow cytometry, or mailed to a reference laboratory for
special studies (such as ploidy, gene amplification studies, or fluo-rescence
in situ hybridization).
5. Freeze a
minimum of 1 cc of tumor tissue in liquid nitrogen. This can be submitted to
refer-ence laboratories for protocol studies or held locally in a tumor bank if
available. Normal tissue should also be frozen. If you have lim-ited tissue,
remember that the frozen section control is often inadequate for permanent
his-tology, yet if it is kept frozen it can be used for these studies.
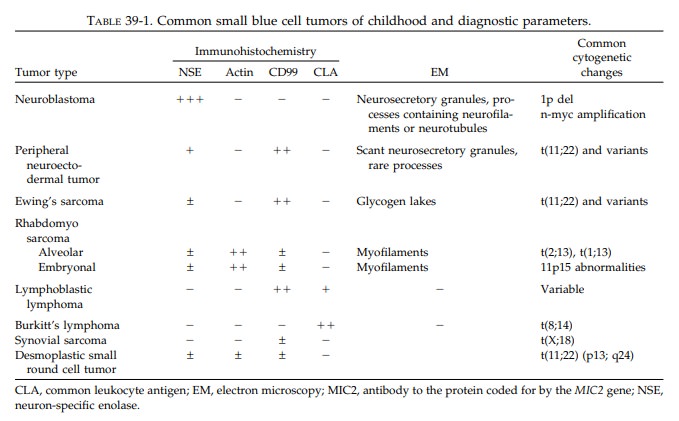
Small Blue Cell Tumors of Childhood
Pediatric
tumors are often embryonal neoplasms showing little or no differentiation by
routinehistology. In particular, at the time of frozen sec-tion, these tumors
appear to be primitive, small, round blue cell tumors. Their diagnosis often
depends on ancillary studies such as immuno-histochemistry, electron
microscopy, and cyto-genetics or molecular genetic analysis. If all pediatric
specimens are processed as delineated in the first section, all necessary
information should be available in a timely fashion. Table 39-1 lists the most
common small blue tumors of child-hood and their pertinent diagnostic features.
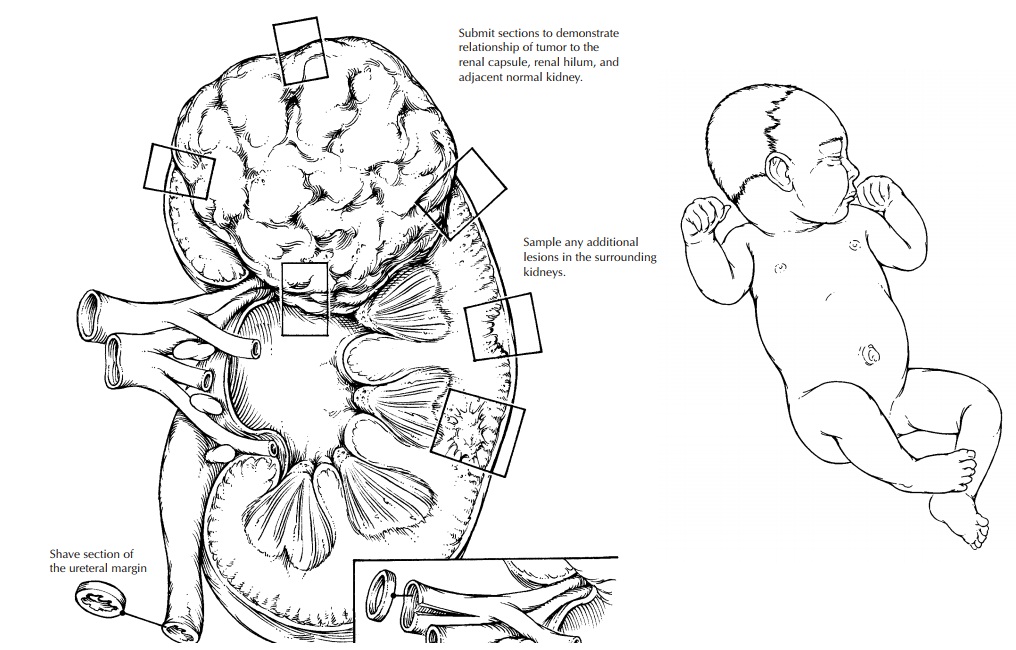
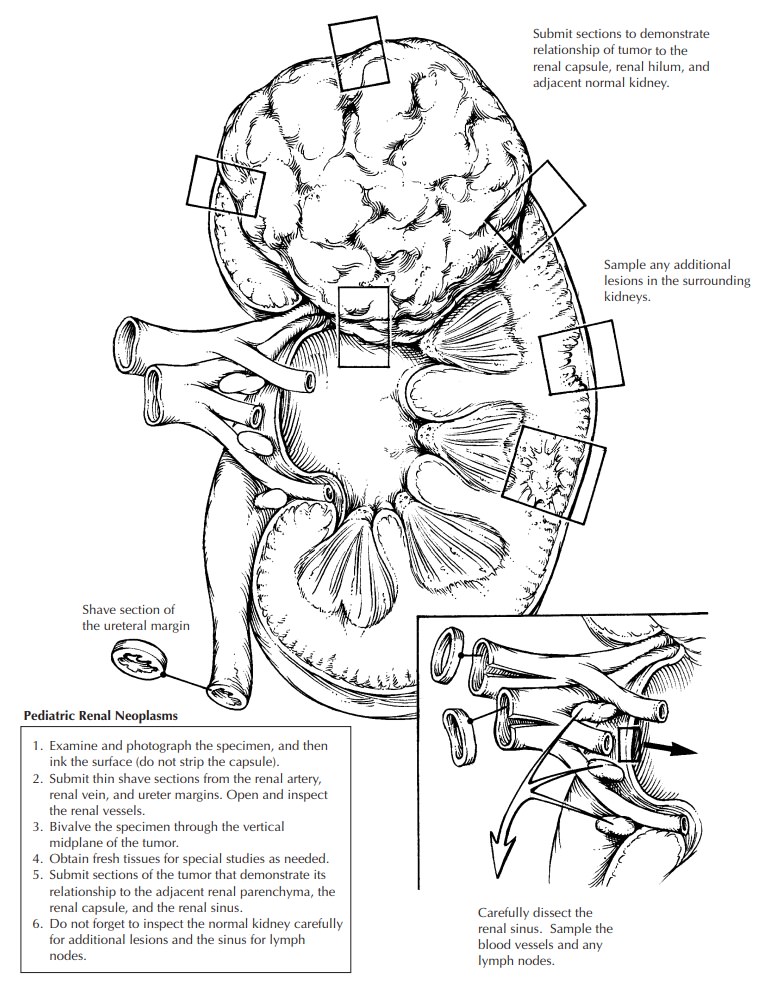
Pediatric Renal Neoplasms
Pediatric
renal tumors are often primarily re-sected and must be carefully processed to
en-sure accurate staging.19Since these tumors are often
bulky and friable and are therefore easily distorted, processing must be
undertaken with care.
1. Photograph
the nephrectomy specimen before bivalving. Carefully examine the contour of the
kidney and the tumor, and identify poten-tial sites of capsular penetration.
2. Ink the
surface (do not strip the capsule).
3. Submit
shave sections of the vascular and ure-teral margins. The renal vein margin is
particu-larly important.
4. Bivalve
the specimen. The kidney should always be bivalved by the pathologist after steps 1–3 are performed, not by
the surgeon and not in the operating room. Choose your plane of incision
carefully, as the placement of the origi-nal incision determines your ability
to docu-ment the relationship between the tumor and kidney, the tumor and the
renal sinus, etc. The incision should be at or near the vertical mid-plane of
the kidney. This cut should avoid sites of capsular penetration if possible.
5. Obtain
fresh tissues needed for special studies (cytogenetics, frozen, etc.), as
outlined pre-viously. Photograph the bivalved specimen.
6. Make
cuts parallel to the initial bivalving inci-sion at 2- to 3-cm intervals.
Submerge the spec-imen in a large container of formalin. If the formalin can be
refrigerated, color preserva-tion will be enhanced and autolysis slowed.
7. After a
few hours or overnight fixation, the remaining sections may be obtained. Two
slides should be prepared from all tissue blocks to expedite the mailing of
slides to the external review pathologist. The majority of the routine tumor
sections should be taken from the periphery of the lesion, showing the
following:
a. Nature of the tumor–kidney junction.
b. Relationship
of the tumor to the renal capsule, particularly in areas of concern for
capsular penetration.
c. Relationship
of the tumor to the renal sinus.
d. Areas
of the tumor that appear different (e.g., necrosis, hemorrhage). Always
in-dicate the exact site from which each sec-tion is taken. This is most easily
done by taking Polaroid or digital photographs. Drawings are often
insuf-ficient.
8. Carefully
section and inspect the normal kid-ney, particularly adjacent to the tumor. These
areas may show microscopic foci of persis-tent embryonal tissue known as
nephrogenic rests, the potential precursor lesion of nephro-blastomas.
9. Carefully
dissect the hilar and perinephric tissues for lymph nodes. Failure to submit
re-gional lymph nodes may render patients ineli-gible for some low-stage
protocols.
Using the above guidelines for submission of blocks, histologic evaluation should then pro-vide the specific tumor diagnosis as well as the stage. The staging currently used for pediatric tumors is provided in Table 39-2.
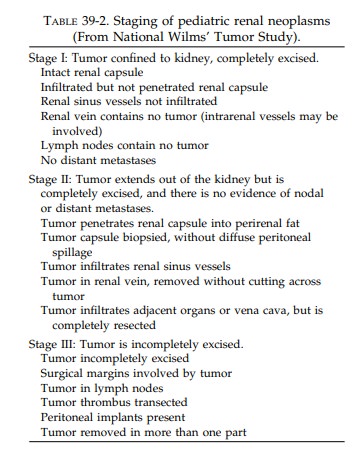
The diagnosis of stage II neoplasms depends on the
identifica-tion of either renal capsular penetration or in-vasion of vessels of
the ‘‘renal sinus.’’ The renal sinus is the principal portal of exit for tumor
cells from the kidney and therefore deserves careful study. The renal sinus is
the concave portion of the kidney that contains much of the pelvicalyceal
system and the principal arteries, veins, lymphat-ics, and nerves that pass
through this sinus. It is largely filled with vascularized adipose tissue. The
renal sinus can be recognized histologically by the fact that the renal cortex
lining the sinus lacks a capsule. A thick capsule surrounds the pelvicalyceal
structures and continues to cover the medullary pyramids. The distinction
between stage I and stage II tumors includes either pene-tration of the renal
capsule or infiltration of ves-sels of the sinus. Some stage I tumors can
distort the renal sinus and protrude with a smoothly encapsulated surface
without invading the soft tissue of the renal sinus. Such tumors do not meet
the criteria for upstaging, unless they show renal capsular penetration.
![]()
Important Issues to Address in Your Report on Pediatric Renal Neoplasms
• What
procedure was performed, and what structures/organs are present?
• What
type of neoplasm is present? The most common diagnoses in children are Wilms
tumor, clear cell sarcoma of kidney, rhabdoid tumor, congenital mesoblastic
nephroma, and renal cell carcinoma. If Wilms tumor, state whether the histology
is favorable or unfavor-able. Unfavorable histology is based on the pres-ence of
cells with nuclei four times the size of surrounding blastemal cells and the
presence of aberrant, multipolar mitotic figures. If unfa-vorable histology
(also called anaplasia) is pres-ent,
comment on its extent (focal or diffuse).
• What is
the size of the tumor (weight and greatest dimension)?
• Are any
margins involved?
• Is the
renal vein involved by tumor?
• Is renal
capsular penetration present?
• Is renal
sinus invasion present?
• Has the
tumor metastasized to regional lymph nodes? Record the number of metastases and
the total number of lymph nodes examined.
Related Topics