Chapter: Microbiology and Immunology: Bacteriology: Haemophilus, Pasteurella, and Actinobacillus
Pathogenesis and Immunity - Haemophilus influenza
Pathogenesis and Immunity
Haemophilus are the obligate bacteria present in the mucousmembranes of humans and certain species of animals. The encapsulated strains, such as strain type b are usually associ-ated with invasive conditions, such as pneumonia, meningitis, septicemia, cellulitis, septic arthritis, etc. The unencapsulated strains primarily cause infections at mucosal surfaces, includ-ing otitis media, conjunctivitis, bronchitis, and sinusitis.
◗ Virulence factors
H. influenzae produces following virulent factors (Table 38-2):Capsular polysaccharide: Capsular polysaccharide is themajor virulence factor in Hib. This polysaccharide capsule, which contains ribose, ribitol, and phosphate, known as PRP, is antiphagocytic. It resists phagocytosis of the bacteria by leu-kocytes. Loss of capsule leads to loss of virulence of the bacteria. Antibodies against this capsule are protective. It promotes bacte-rial phagocytosis and complement-mediated bactericidal activi-ties. These antibodies are produced during natural infection or on vaccination with purified PRP or passive transfer of maternal antibodies from mother to children. The risk of meningitis and epiglottitis is much higher in patients with no anti-PRP anti-bodies or in patients with low level of complement.
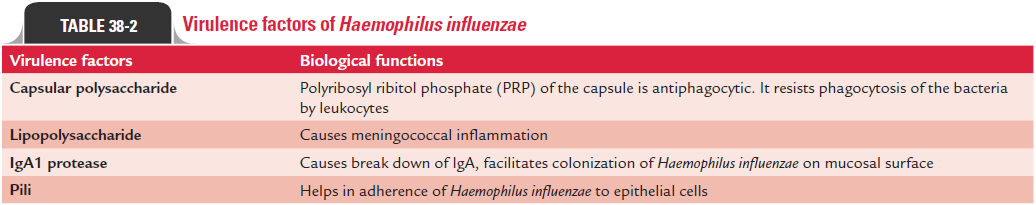
Lipid A lipopolysaccharide: This has been suggested to beresponsible for inducing meningococcal inflammation in humans as demonstrated in experimental animal models.
IgA1 protease: Both encapsulated and nonencapsulatedH. influenzae produce IgA1 protease that specifically splits heavychain of IgA1. Break down of immunoglobulin IgA facilitates colonization of H. influenzae on mucosal surface.
Pili: Pili help in adherence ofH. influenzaeto epithelial cells.
◗ Pathogenesis of H. influenzae infection
H. influenzae enters the human host by respiratory route. Piliand nonpilous adhesions of the bacteria mediate colonization of H. influenzae in the oropharynx or nasopharynx. Lipid A lipo-polysaccharide impairs ciliary function, leading to damage of the respiratory mucosa. A large bacterial load or the presence of concomitant viral infection can potentiate the infection of the bacteria that invade the mucosa and enter the blood stream.
The presence of antibodies, complement components, and phagocytes determines the clearance of bacteremia. The absence of anti-PRP antibodies contributes to bacterial multiplication. Subsequent high-grade bacteremia leads to dissemination of bacteria to various sites including meninges, subcutaneous tissues, joints, and even pleura and pericardium.
Bacteremia usually precedes Hib meningitis and other inva-sive Hib diseases. Direct extension of infection from the sinuses or ears is rare. The magnitude and duration of bacteremia are the primary determinants of central nervous system (CNS) invasion, which occurs through the choroid plexus.
Meningitis occurs as a result of inflammation, edema, and increased cerebrospinal fluid (CSF) pressure. Brain parenchy-mal invasion is rare.
Noncapsulated or nontypable influenza strains, which colonize up to 80% of individuals, cause infection by direct extension from the colonized sites. Spread of bacteria by direct extension to sinuses causes sinusitis, to the Eustachian tube causes otitis media, and down the respiratory tract results in bronchitis and pneumonia.
◗ Host immunity
Antibodies directed against the PRP component of capsule play a primary role in conferring immunity. Newborns have a low risk of infection because of the passive transfer of maternal antibodies. By age of 5 years, most children have naturally acquired antibodies.
Related Topics