Chapter: Organic Chemistry: Reactions and mechanisms
Organic Chemistry Reactions
REACTIONS
Key Notes
Bond formation
Most
organic reactions take place between nucleophiles and electrophiles, where the
nucleophilic center of the nucleophile forms a bond to the electrophilic center
of the electrophile.
Classification of reactions
Reactions
can be classified as acid/base reactions, functional group trans-formations or
as carbon–carbon bond formations. Reactions can also be classified according to
the process or mechanism taking place and these are specific for particular
functional groups.
Bond formation
Synthetic organic chemistry is about creating
complex molecules from simple starting materials – a process which may involve
many different reactions. Designing a synthesis is a bit like chess. A grand
master has to know the pieces and the moves that can be made before planning a
game strategy. As far as an organic chemist is concerned, he/she has to know the
molecules and the sort of reactions which can be carried out before planning a
synthetic ‘game strategy’.
Inevitably, there is a lot of memory work
involved in knowing reactions, but there is a logic involved as well.
Basically, most reactions involve electron-rich molecules forming bonds to
electron deficient molecules (i.e. nucleophiles forming bonds to
electrophiles). The bond will be formed specifically between the nucleophilic
center of the nucleophile and the electrophilic center of the electrophile.
Classification of reactions
There are a large number of reactions in
organic chemistry, but we can simplify the picture by grouping these reactions
into various categories. To begin with, we can classify reactions as being:
·
acid/base reactions;
·
functional group transformations;
·
carbon–carbon bond formations.
The first category of reaction is relatively simple and involves the reaction of an acid with a base to give a salt. These reactions are covered in Section G. The second category of reaction is where one functional group can be converted into another. Normally these reactions are relatively straightforward and proceed in high yield. The third category of reactions is extremely important to organic chemistry since these are the reactions which allow the chemist to construct complex molecules from simple starting materials. In general, these reactions are the most difficult and temperamental to carry out. Some of these reactions are so important that they are named after the scientists who developed them (e.g. Grignard and Aldol reactions).
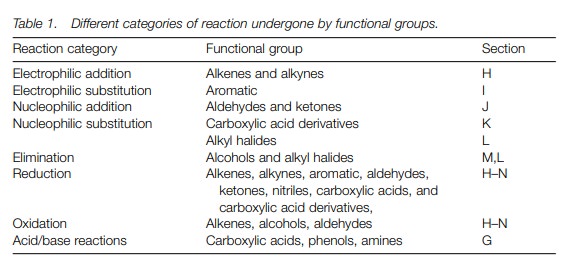
Another way of categorizing reactions is to
group similar types of reactions together, depending on the process or
mechanism involved. This is particularly useful since specific functional
groups will undergo certain types of reaction cat-egory. Table 1 serves as a summary of the types of reactions which
functional groups normally undergo.
Related Topics