Chapter: Organic Chemistry: Reactions and mechanisms
Organic Chemistry Mechanisms
MECHANISMS
Key Notes
Definition
A
mechanism describes how a reaction takes place by showing what is hap- pening
to valence electrons during the formation and breaking of bonds.
Curly arrows
Curly
arrows are used to show what happens to valence electrons during the making and
breaking of bonds. They always start from the source of two electrons (i.e. a
lone pair of electrons on an atom or the middle of a bond about to be broken).
They always point to where the valence electrons will end up. If the electrons
end up as a lone pair of electrons on an atom, the arrow points to that specific
atom. If the electrons are being used to form a new bond, the arrow points to
where the center of the new bond will be formed.
Half curly arrows
Half
curly arrows are used to show the movement of single electrons dur- ing radical
reactions. Bond breaking
during a radical
reaction involves homolytic
cleavage where the bonding electrons move to different atoms.
However,
most reactions in organic chemistry involve heterolytic cleavage where the
bonding electrons move as a pair onto one atom and not the other.
Definition
An understanding of electrophilic and
nucleophilic centers allows a prediction of where reactions might occur but not
what sort of reaction will occur. In order to understand and predict the
outcome of reactions, it is necessary to understand what goes on at the
electronic level. This process is known as a mechanism.
A mechanism is the ‘story’ of how a reaction takes place. It explains how mole- cules react together to give the final product. The mechanism tells us how bonds are formed and how bonds are broken and in what order. It explains what is hap- pening to the valence electrons in the molecule since it is the movement of these electrons which result in a reaction. Take as a simple example the reaction between a hydroxide ion and a proton to form water (Fig. 1). The hydroxide ion is a nucleophile and the proton is an electrophile. A reaction takes place between the nucleophilic center (the oxygen) and the electrophilic center (the hydrogen) and water is formed. A new bond has been formed between the oxygen of the hydroxide ion and the proton. The mechanism looks at what happens to the electrons.
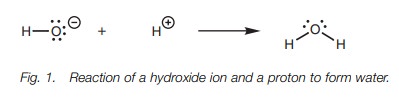
In this example, a lone pair of electrons from
oxygen is used to form a bond to the proton. By doing so, the oxygen
effectively ‘loses’ one electron and the proton effectively gains one electron.
As a result, the oxygen loses its negative charge and the proton loses its
positive charge.
Curly arrows
Explaining what happens to all the valence
electrons during a reaction mechanism can be rather long-winded if you are
trying to explain it all in words. Fortunately, there is a diagrammatic way of
showing the same thing – using curly arrows. For example, the mechanism
described above can be explained by using a curly arrow to show what happens to
the lone pair of electrons (Fig. 2).
In this case, the arrow starts from a lone pair of electrons on the oxygen (the
source of the two electrons) and points to where the center of the new bond will be formed.
In some textbooks, you may see the arrow
written directly to the proton (Fig. 3).
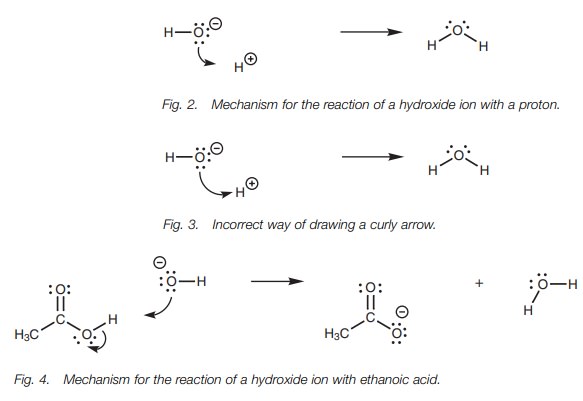
Formally, this is incorrect. Arrows should only
be drawn directly to an atom if the electrons are going to end up on that atom
as a lone pair of electrons.
The following rules are worth remembering when
drawing arrows:
● curly arrows show the movement of electrons,notatoms;
● curly arrows start from the source of two electrons (i.e. a lone
pair of electrons on an atom or the middle of a bond which is about to be
broken);
● curly arrows point to anatomif
the electrons are going to end up as a lone pair on that atom;
● curly arrows point to where a new bond will be formed if the
electrons are being used to form a new bond.
The mechanism (Fig. 4) explains what happens when a hydroxide ion reacts with a carboxylic acid and is a demonstration of how arrows should be drawn. One of the lone pairs of electrons on the hydroxide ion is used to form a bond to the acidic proton of the carboxylic acid. The curly arrow representing this starts from a lone pair of electrons and points to the space between the two atoms to show that a bond is being formed.
At the same time as this new bond is being
formed, the O–H bond of the car-boxylic acid has to break. This is because the
hydrogen atom is only allowed one bond. The electrons in this bond end up on
the carboxylate oxygen as a third lone pair of electrons. The arrow
representing this starts from the center
of the bond being broken and points directly to the atom where the electrons
will end up as a lone pair.
Notice also what happens to the charges. The
negatively charged oxygen of the hydroxide ion ends up as a neutral oxygen in
water. This is because one of the oxygen’s lone pairs is used to form the new
bond. Both electrons are now shared between two atoms and so the oxygen
effectively loses one electron and its negative charge. The oxygen in the
carboxylate ion (which was originally neutral in the carboxylic acid) becomes
negatively charged since it now has three lone pairs of electrons and has
effectively gained an extra electron.
Half curly arrows
Occasionally reactions occur which involve the
movement of single electrons rather than pairs of electrons. Such reactions are
known as radical reactions. For
example, a chlorine molecule can be split into two chlorine radicals on
treatment with light. One of the original bonding electrons ends up on one
chlorine radical and the second bonding electron ends up on the other chlorine
radical. The movement of these single electrons can be illustrated by using
half curly arrows rather than full curly arrows (Fig. 5).

This form of bond breaking is known as a homolytic cleavage. The radical atoms
obtained are neutral but highly reactive species since they have an unpaired
valence electron.
There are some important radical reactions in
organic chemistry, but the major-ity of organic reactions involve the heterolytic cleavage of covalent bonds
where electrons move together as a pair (Fig.
6).
Related Topics