Chapter: Organic Chemistry: Nucleophiles and electrophiles
Nucleophiles and electrophiles: Organic structures
ORGANIC STRUCTURES
Key Notes
Alkanes
Alkanes
have no nucleophilic or electrophilic centers and are unreactive.
Polar functional groups
The
nucleophilic and electrophilic centers of functional groups can be iden-tified
by identifying polar bonds. Bonds such as C–H and C–C are consid-ered to be
nonpolar. Carbon bonded to oxygen or halogen is an electrophilic center. Some
electrophilic and nucleophilic centers are weak and are not usually important.
Unsaturated Unsaturated
Alkenes,
alkynes, and aromatic rings are nucleophiles. The multiple bonds in these
functional groups represent an area of high electron density and are therefore
nucleophilic centers.
Alkanes
Alkanes are made up of carbon–carbon and carbon–hydrogen
single bonds and are unreactive compounds. This is because C–C and C–H bonds
are covalent in nature and so there are no electrophilic or nucleophilic
centers present. Since most reagents react with nucleophilic or electrophilic
centers, alkanes are unreactive molecules.
Polar functional groups
It is possible to identify the nucleophilic and
electrophilic centers in common functional groups, based on the relative
electronegativities of the atoms present.
The following guidelines are worth remembering:
·
C–H and C–C bonds are covalent. Therefore, neither carbon nor
hydrogen is a nucleophilic or electrophilic center;
· nitrogen is immediately to the right of carbon in the periodic table. The nitro-gen is more electronegative but the difference in electronegativity between these two atoms is small and so the N–C bond is not particularly polar. There-fore, the carbon atom can usually be ignored as an electrophilic center;
·
N–H and O–H bonds are polar covalent. Nitrogen and oxygen are
strong nucleophilic centers. Hydrogen is a weak electrophilic center;
·
C=O, C=N and C==N bonds are polar covalent. The O and N are
nucleophilic centers and the carbon is an electrophilic center;
·
C–O and C–X bonds (X halogen) are polar covalent. The oxygen atom
is moderately nucleophilic whereas the halogen atom is weakly nucleophilic. The
carbon atom is an electrophilic center.
Using the above guidelines, the nucleophilic
and electrophilic centers of the com-mon functional groups can be identified,
where atoms having a slightly negative charge are nucleophilic centers and
atoms having a slightly positive charge are electrophilic centers (Fig. 1).
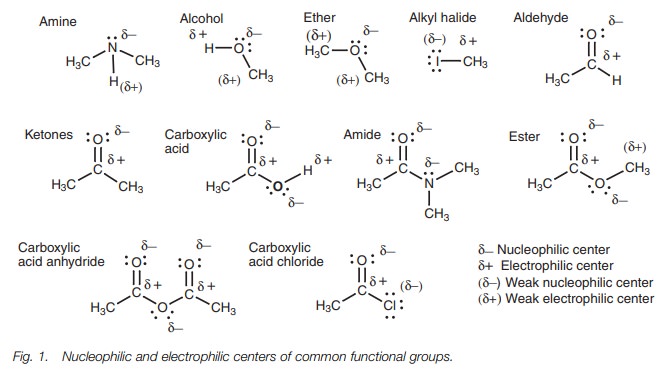
Not all the nucleophilic and electrophilic
centers are of equal importance. For example, a nitrogen atom is more
nucleophilic than an oxygen atom. Also halogen atoms are very weakly
nucleophilic and will not usually react with electrophiles if there is a
stronger nucleophilic center present. Hydrogen atoms attached to halogens are
more electrophilic than hydrogen atoms attached to oxygen. Hydro-gen atoms
attached to nitrogen are very weakly electrophilic.
Taking this into account, some functional
groups are more likely to react as nucleophiles while some functional groups
are more likely to react as electro-philes. For example, amines, alcohols and
ethers are more likely to react as nucle-ophiles, since they have strong
nucleophilic centers and weak electrophilic centers. Alkyl halides are more
likely to react as electrophiles since they have strong electrophilic centers
and weak nucleophilic centers. Aldehydes and ketones can react as nucleophiles
or electrophiles since both electrophilic and nucleophilic centers are strong.
Some functional groups contain several
nucleophilic and electrophilic centers. For example, carboxylic acids and their
derivatives fall into this class and so there are several possible centers
where a nucleophile or an electrophile could react.
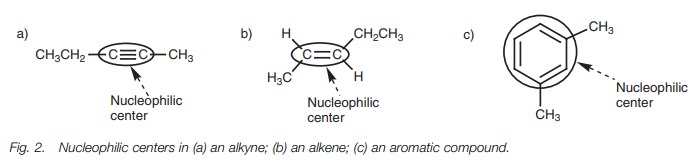
Unsaturated hydrocarbons
Not
all functional groups
have polar bonds.
Alkenes, alkynes, and
aromatic compounds are examples of functional groups which have covalent
multiple bonds. The space between the multiple bonded carbons is rich in
electrons and is therefore nucleophilic. Thus, the nucleophilic center in these
molecules is not a specific atom, but the multiple bond (Fig. 2)!
Related Topics