Chapter: Organic Chemistry: Nucleophiles and electrophiles
Nucleophiles and electrophiles: Charged species
CHARGED SPECIES
Key Notes
Anions
Negatively
charged ions with lone pairs of electrons are nucleophiles. The atom bearing
the negative charge is the nucleophilic center.
Cations
Positively
charged ions are electrophiles. The atom bearing the positive charge is the
electrophilic center.
Relative nucleophilicity
In a
series of anions, the relative nucleophilic strength matches their relative
basicity if the nucleophilic center is the same atom. The same holds true for
anions where the nucleophilic center is an atom from the same row of the
periodic table. In protic solvents, anions having large nucleophilic centers
(atoms lower down the periodic table) are less solvated and are stronger
nucleophiles. In aprotic solvents nucleophilic strengths more closely match
relative basicity.
Anions
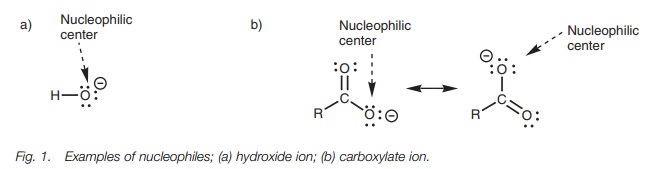
A negatively charged molecule such as the
hydroxide ion (Fig. 1) is electron
rich and acts as a nucleophile. The atom which bears the negative charge and a
lone pair of electrons is the nucleophilic center, which in the case of the
hydroxide ion is the oxygen atom. Some ions (e.g. the carboxylate ion) are able
to share the negative charge between two or more atoms through a process known
as delocalization. In this case, the
negative charge is shared between both oxygenatoms and so both of these atoms
are nucleophilic centers (Fig. 1).
Cations.
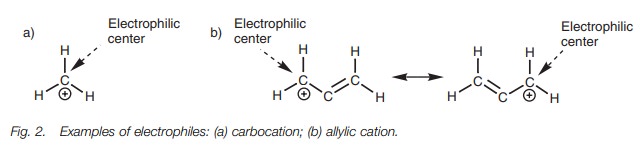
A positively charged ion is electron deficient and acts as an electrophile. The atom which bears the positive charge is the electrophilic center. In the case of a carbocation (Fig. 2), this is the carbon atom. Some molecules (e.g. the allylic cation) are able to delocalize their positive charge between two or more atoms in which case all the atoms capable of sharing the charge are electrophilic centers (Fig. 2).
Relative nucleophilicity
In a series of anions, nucleophilic strength
parallels basicity if the nucleophilic center is the same atom. For example the
nucleophilic strengths of the following oxygen compounds (RO->>HO-
>>RCO2-) matches their order of basicity. The same
holds true for anions where the nucleophilic center is an element in the same
row of the periodic table (e.g. C,N,O,F). Thus, the order of nucleophilic- ity
of the following anions (R3C->R2N-
> RO- > F-) is the same as their order of basicity.
This trend is related to the electronegativities of these atoms. The more
electronegative the atom (e.g. F), the more tightly it holds on to its
electrons and the less available these electrons are for forming new bonds
(less nucleophilic).
The story becomes more complex if we compare
anions having nucleophilic centers from different parts of the periodic table.
Here, relative nucleophilicity does not necessarily match relative basicity.
This is because the solvent used in a reaction has an important effect. In
protic solvents such as water or alcohol, the stronger nucleophiles are those
which have a large nucleophilic center, that is, an atom lower down the
periodic table (e.g. S is more
nucleophilic than O but is less basic).
This is because protic solvents can form hydrogen bonds to the anion.
The smaller the anion, the stronger the
solvation and the more difficult it is for the anion to react as a nucleophile.
The order of nucleophilicity of some common
anions in protic solvents is as follows: SH- > CN- >
I- > OH- > N3- > Br- >
CH3CO2- > Cl -> F- .
When an organic solvent is used which is
incapable of forming hydrogen bonds to the anion (e.g. DMF or DMSO; Fig. 3),
the order of nucleophilicity changes to more closely match that of basicity.
For example, the order of nucleophilicity of the halides in DMSO is F ->
Cl -> Br -> I- .
Related Topics