Chapter: Pharmaceutical Drug Analysis: Non-Aqueous Titrations
Non-Aqueous Titrations: Methodology
METHODOLOGY
For non-aqueous titrations, the following four steps are usually taken into
consideration, namely :
(i) Preparation
of 0.1 N Perchloric acid,
(ii)
Standardization of 0.1 N Perchloric Acid,
(iii) Choice of
Indicators, and
(iv) Effect of
Temperature on Assays.
1. PREPARATION OF 0.1 N PERCHLORIC ACID
Materials Required : 8.5 ml of perchloric acid
(70.0 to 72.0%) ; 1 Litre of glacial acetic acid ; 30 ml of acetic anhydride.
Procedure : Gradually mix 8.5 ml of
perchloric acid to 900 ml of glacial acetic acid with vigorous and continuous stirring. Now add 30 ml
acetic anhydride and make up the volume to 1 litre with glacial acetic acid and
allow to stand for 24 hours before use.
The acetic anhydride reacts with the water (approx. 30%)
in perchloric acid and some traces in glacial acetic acid thereby making the
resulting mixture practically anhydrous. Thus, we have :
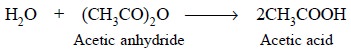
Precautions : The following precautions must
be observed :
(a) Perchloric
acid is usually available as a 70 to 72% mixture with water (sp. gr. 1.6). It
usually undergoes a spontaneous explosive decomposition and, therefore, it is
available always in the form of a solution.
(b) Conversion
of acetic anhydride to acetic acid requires 40-45 minutes for its completion.
It being an exothermic reaction, the solution must be allowed to cool to room
temperature before adding glacial acetic acid to volume,
(c)
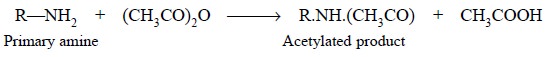
Avoid adding an excess of acetic anhydride especially
when primary and secondary amines are to be assayed, because these may be
converted rapidly to their corresponding acetylated non-basic products :
(d) Perchloric
acid is not only a powerful oxidising agent but also a strong acid. Hence, it
must be handled very carefully.
Perchloric acid has a molecular weight of 100.46 and 1 L
of 0.1 N solution shall contain 1 /10th the equivalent weight or 10.046 g. To
prepare 1 L of standard perchloric acid solution, it requires 8.5 ml (sp. gr.
1.6) volume and a purity of 72% which will calculate out as 9.792 g of HClO4.
2. STANDARDIZATION OF 0.1 N PERCHLORIC ACID
Alkaline earth (e.g.,
Mg, Ca, Ba), and alkali (e.g., Na, K,
Rb), salts of organic acids behave as bases in acetic acid solution :
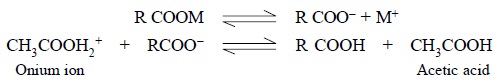
In usual practice, potassium hydrogen phthalate (or
potassium biphthalate, KHC8H4O4) is employed
as a standardizing agent for acetous perchloric acid. The reaction may be
expressed as follows :
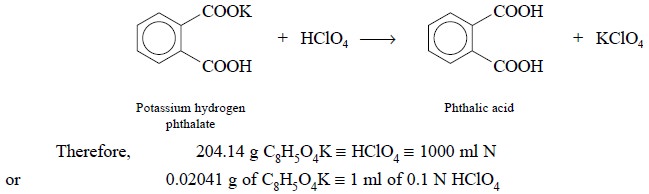
Procedure : Weigh accurately about 0.5 g
of potassium hydrogen phthalate in a 100 ml conical flask. Add 25 ml of glacial acetic acid and attach a reflux condenser
fitted with a silica-gel drying tube. Warm until the salt gets dissolved
completely. Cool and titrate with 0.1 N perchloric acid by making use of either
of the following two indicators :
(a) acetous
crystal violet-2 drops, end point Blue to Blue-Green (0.5% w/v)
(a)
acetous oracet blue B-2 drops, end point Blue to Pink.
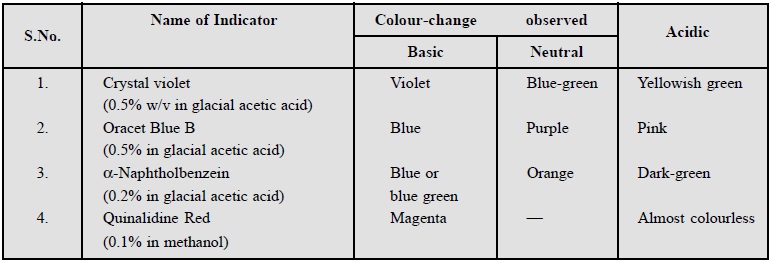
3. CHOICE OF INDICATORS
A number of indicators stated below are commonly used in
non-aqueous titrations. It is, however, necessary to mention here that the same
indicator must be used throughout for carrying out the standardiza-tion,
titration and neutralization of mercuric acetate solution.
4. EFFECT OF TEMPERATURE ON ASSAYS
Generally, most non-aqueous solvents possess greater
coeffcients of expansion as compared to water, which is why small differences
in temperature may afford significant and appreciable errors that can be
eliminated by the application of appropriate correction factors. Hence, it is
always advisable to carry out standardization and titration preferably at the
same temperature. In a situation where these temperature parameters cannot be
achieved, the volume of titrant may be corrected by the application of the
following formula :
Vc =
V [1 + 0.001 (tl + t2)]
where, Vc = Corrected
volume of titrant,
V = Volume of titrant measured,
tl = Temperature at which titrant was standardized, and
t2 = Temperature at which titration was performed.
Related Topics