Chapter: Pharmaceutical Drug Analysis: Non-Aqueous Titrations
Alkalimetry in Non-Aqueous Titrations
ALKALIMETRY IN NON-AQUEOUS TITRATIONS
A plethora of weakly acidic pharmaceutical substances may
be titrated effectively by making use of a suitable non-aqueous solvent with a
sharp end-point. The wide spectrum of such organic compounds in-clude :
anhydrides, acids, amino acids, acid halides, enols (viz., barbiturates), xanthines, sulphonamides, phenols, imides and
lastly the organic salts of inorganic acids.
However, a weak inorganic acid e.g., boric acid, can be estimated conveniently employing
ethylenediamine as the non-aqueous solvent.
1. Preparation of 0.1 N Potassium Methoxide in Toluene-Methanol
Materials Required : Absolute methanol : 40 ml ;
dry toluene : 50 ml ; potassium metal : 4 g.
Procedure : Add into a dry flask, a
mixture of methanol (40 ml) and dry toluene (50 ml) and cover it loosely. Carefully add freshly cut
pieces of potassium metal to the above mixture gradually with constant shaking.
After complete dissolution of potassium metal, add enough absolute methanol to
yield a clear solution. Toluene 50 ml is added with constant shaking until the
mixture turns hazy in appearance. The process is repeated by the alternate
addition of methanol and benzene until 1 litre of solution is obtained, taking
care to add a minimum volume of methanol to give a visible clear solution.
1.1. Preparation of 0.1 N Sodiun
Methoxide
It is prepared exactly in a similar manner as for 0.1 N
Potassium Methoxide, using 2.3 g of freshly-cut sodium in place of potassium.
1.2. Preparation of 0.1 N Lithium
Methoxide
It is prepared as for 0.1 N Potassium Methoxide, but
using 0.7 g of lithium in place of potassium.
2. Standardization of 0.1 N Methoxide Solution
Materials Required : Dimethylformamide (DMF) : 10
ml ; thymol blue (0.3% in MeOH) ;
0.1 N lithium methoxide in toluene-methanol ; benzoic acid : 0.6 g.
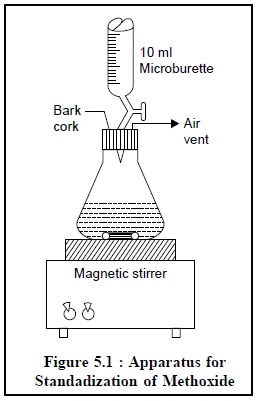
Procedure : The apparatus shown in Figure
5.1, is employed for the
standardization of 0.1 N methoxide solution. Transfer 10 ml of DMF in a conical
flask and add to it 3 to 4 drops of thymol blue and first neutralize the acidic
impurities present in DMF by titrating with 0.1 N lithium methoxide in
toluene-methanol. Quickly introduce 0.06 g of benzoic acid and titrate
immediately with methoxide in toluene-methanol.
Caution : Care must be taken
to avoid contamination of neutralized liquid with atmospheric carbon dioxide.
Equations : The various equations involved in the above operations
are summarized as stated below:
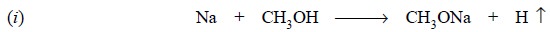
Interaction between sodium metal and methanol is an
exothermic reaction and hence, special care must be taken while adding the
metal into the dry solvent in small lots at intervals with adequate cooling so
as to keep the reaction well under control.
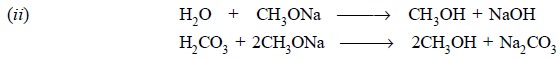
The clear solution of sodium methoxide must be kept away
from moisture and atmospheric CO2 as far as possible so as to avoid
the above two chemical reactions that might ultimately result into the
formation of turbidity.
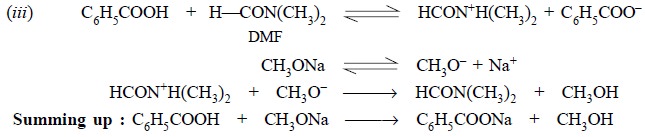
Step 1 : It shows the solution of
benzoic acid (primary standard) in DMF,
Step 2 : It depicts ionization of
sodium methoxide,
Step 3 : It illustrates the interaction
between the solvated proton and the methylated ion.
In summing up, the net reaction in the process of
standardization has been expressed. The interaction between the water in the solvent
(DMF) and the titrant is equivalent to the volume of sodium methoxide consumed
by DMF or may be considered as a blank determination.
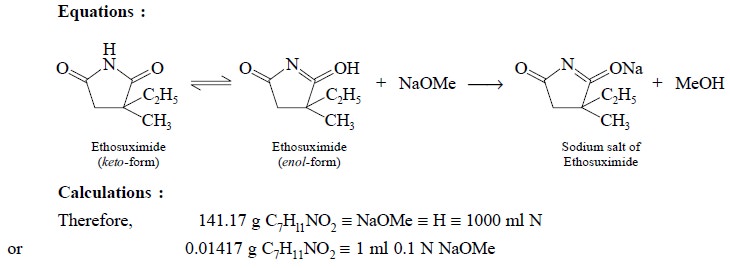
2.1. Ethosuximide
Materials Required : Ethosuximide : 0.2 g ;
dimethylformamide : 50 ml ; azo-violet (0.1% w/v in DMF) : 2 drops ; sodium
methoxide 0.1 N.
Procedure : Weigh accurately about 0.2 g,
dissolve in 50 ml of dimethylformamide, add 2 drops of azo-violet solution and tirate with 0.1 N sodium methoxide to a
deep blue end point, taking precautions to prevent absorption of atmospheric
carbon dioxide. Perform a blank determination and make any necessary
correction. Each ml of 0.1 N sodium methoxide is equivalent to 0.01412 g of C7H11NO2.
Equations :
3. Cognate Assays
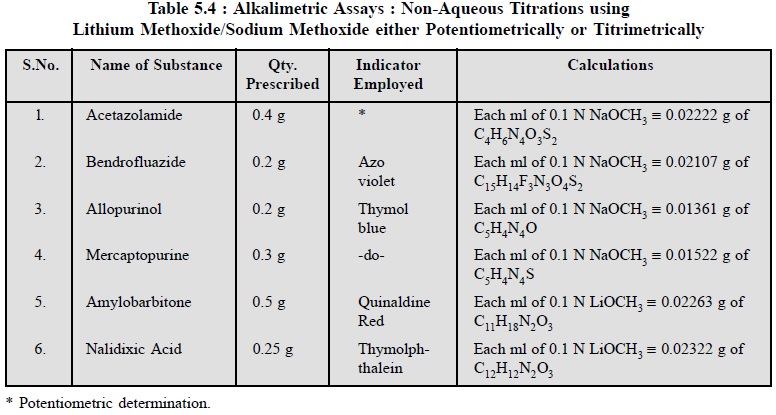
The following determinations as stated in Table 5.4 may
be carried out effectively by using 0.1 N sodium hydroxide either
titrimetrically using an appropriate indicator or potentiometrically :
4. Tetrabutylammonium Hydroxide
The alkalimetry in non-aqueous titrations may also be
carried out efficiently by using tetrabutylammonium hydroxide along with an
appropriate indicatior.
4.1. Preparation of 0.1 N
Tetrabutylammonium Hydroxide in Toluene-Methanol
Materials Required : Tetrabutylammonium iodide : 40
g ; absolute methanol : 90 ml ; silver oxide : 25 g ; dry toluene : 150 ml.
Procedure : Carefully dissolve 40 g of
tetrabutylammonium iodide (Bu4NI) in 90 ml of absolute methanol, add to it 20 g of finely powdered purified
silver oxide and finally shake the mixture thoroughly for 1 hour. Centrifuge
about 2-3 ml of the resultant mixture and test for iodide in the supernatant
liquid. In case, it gives a positive test, add about 2 g more of silver oxide
and shake for an additional period of 30 minutes. The said method may be
repeated until the supernatant liquid obtained is completely free from iodide.
The mixture thus obtained is filtered through a fine sintered glass filter and
finally rinse the container with 3 portions, each of 50 ml of dry toluene.
These washings may be added to the filtrate and the final volume is made upto 1
litre with dry toluene. The clear solution may be flushed with CO2-free
nitrogen for at least five minutes and duly protected from both CO2
and moisture during storage.
Equation :
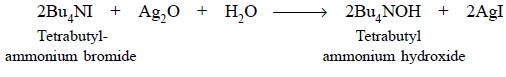
4.2. Standardization of 0.1 N Tetrabutylammonium Hydroxide
Materials Required : Benzoic acid : 60 mg ;
dimethylbromide : 10 ml ; thymol blue solution (0.3% w/v in methanol) ; 0.1 N tetrabutylammonium hydroxide.
Procedure : Accurately weigh about 60 mg
of benzoic acid into 10 ml of previously neutralized dimethyl formamide to the blue colour of thymol blue (3 drops) by
titration against 0.1 N tetrabutylammonium hydroxide. Allow the benzoic acid to
dissolve gradually and completely and titrate with 0.1 N tetrabutylammonium
hydroxide preferably in an atmosphere of CO2-free nitroaen.
Calculations :
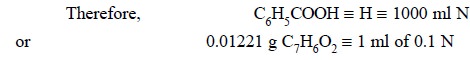
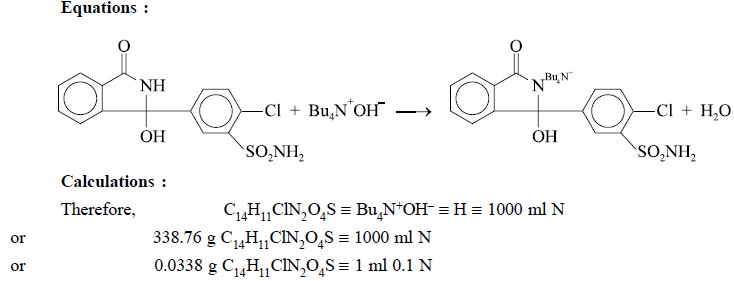
4.3. Chlorthalidone
Materials Required : Chlorthalidone : 0.3 g ;
pyridine (dehydrated) : 50 ml ; 0.1 N tetrabutylammonium hydroxide.
Procedure : Weigh accurately about 0.3 g
and dissolve in 50 ml of dehydrated pyridine. Titrate with 0.1 N tetrabutylammonium hydroxide, determining the end point
potentiometrically and protecting the solution and titrant from atmospheric
carbon dioxide throughout the determination. Perform a blank determination and
make any necessary correction. Each ml of 0.1 N tetrabutylammonium hydroxide is
equivalent to 0.03388 g of Cl4H1lClN2O4S.
Equations :
4.4. Cognate Assays
The following pharmaceutical substances may be assayed by
employing tetrabutylammonium hydroxide either by using a suitable indicator
titrimetrically or potentiometrically as given in Table 5.5.
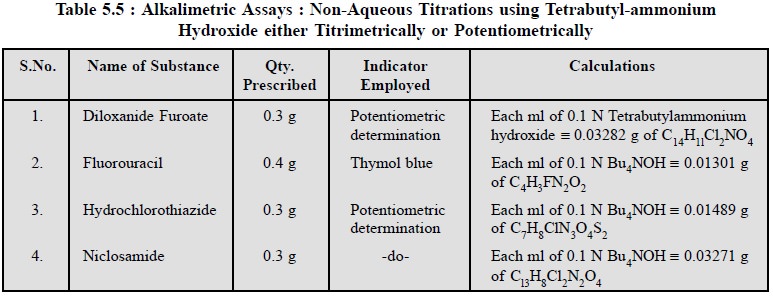
The assay of the aforesaid pharmaceutical substances with
tetrabutylammonium hydroxide is on a mole-for-mole basis. As these are
monobasic acids in character, therefore, they react quantitatively in a
non-aqueous media with the base titrant, employing typical acid-base indicators
to detect the end-points.
Related Topics