Chapter: Clinical Anesthesiology: Clinical Pharmacology: Local Anesthetics
Mechanisms of Local Anesthetic Action
Local Anesthetics
Local and regional anesthesia and
analgesia techniques depend on a group of drugs—local anesthetics—that
transiently inhibit sensory, motor, or autonomic nerve function, or a
combination of these functions, when the drugs are injected or applied near
neural tissue.
MECHANISMS OF LOCAL ANESTHETIC ACTION
Neurons
(and all other living cells) maintain a rest-ing membrane potential of −60 to
−70 mV by active transport and passive diffusion of ions. The electro-genic,
energy consuming sodium–potassium pump (Na+-K+-ATPase) couples the transport of
three sodium (Na) ions out of the cell for every two potas-sium (K) ions it
moves into the cell. This creates an ionic disequilibrium (concentration
gradient) that favors the movement of K ions from an intracellular to an
extracellular location, and the movement of Na ions in the opposite direction.
The cell membrane is normally much more “leaky” to K ions than to Na ions, so a
relative excess of negatively charged ions (anions) accumulates
intracellularly. This accounts for the negative resting potential difference
(–70 mV polarization).
Unlike
most other types of tissue, excitable cells (eg, neurons or cardiac myocytes)
have the capabil-ity of generating action
potentials. Membrane-bound, voltage-gated Na channels in peripheral nerve
axons can produce and transmit membrane depolarizations following chemical,
mechanical, or electrical stimuli. When a stimulus is sufficient to depolarize
a patch of membrane, the signal can be transmitted as a wave of depolarization
along the nerve membrane (an impulse). Activation of volt-age-gated Na channels
causes a very brief (roughly 1 msec) change in the conformation of the channel,
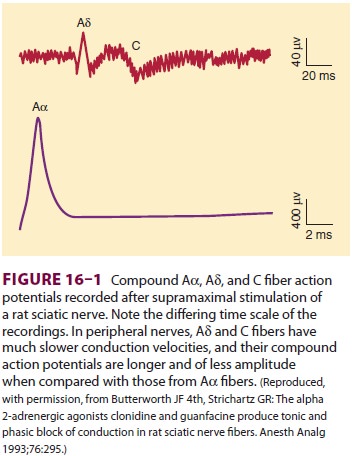
allowing an influx of Na ions and generating an action potential ( Figure 16–1).
The increase in Na permeability causes temporary depolarization of the membrane
potential to +35 mV. The Na current is brief and
is terminated by inactivation of voltage-gated Na channels, which do not
conduct Na ions. Subsequently the membrane returns to its resting potential.
Baseline concentration gradients are maintained by the sodium–potassium pump, and
only a minuscule number of Na ions pass into the cell during an action
potential.Na channels are membrane-bound proteinsthat are composed of one large
α
subunit, through which Na ions pass, and one or two smaller subunits.
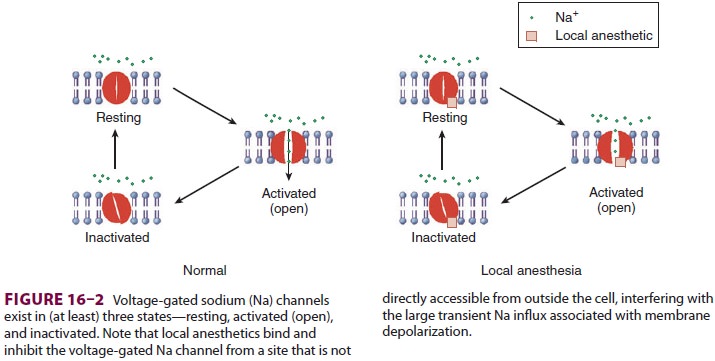
Voltage-gated Na channels exist in (at least) three states—resting
(nonconducting), open
(conducting),
and inactivated (nonconducting) (Figure 16–2). Local anesthetics bind a
specific region of the α
subunit and inhibit voltage-gated Na channels, preventing channel activation
andinhibiting the Na influx associated with membrane depolarization. Local
anesthetic binding to Na chan-nels does not alter the resting membrane
potential. With increasing local anesthetic concentrations, an increasing
fraction of the Na channels in the mem-brane bind a local anesthetic molecule
and cannot conduct Na ions. As a consequence, impulse con-duction slows, the
rate of rise and the magnitude of the action potential decrease, and the
threshold for excitation and impulse conduction increases progressively. At
high enough local anesthetic con-centrations and with a sufficient fraction of
local anesthetic-bound Na channels, an action potential can no longer be
generated and impulse propagation is abolished. Local anesthetics have a
greater affin-ity for the channel in the open or inactivated state than in the
resting state. Local anesthetic binding to open or inactivated channels, or
both, is facilitated by depolarization. The fraction of Na channels that have bound
a local anesthetic increases with fre-quent depolarization (eg, during trains
of impulses). This phenomenon is termed use-dependent block. Put another way,
local anesthetic inhibition is both voltage and frequency dependent, and is
greater when nerve fibers are firing rapidly than with infre-quent
depolarizations.
Local
anesthetics may also bind and inhibit calcium (Ca), K, transient receptor
potential
vanilloid 1 (TRPV1), and many other
channels and receptors. Conversely, other classes of drugs, most notably
tricyclic antidepressants (amitriptyline), meperidine, volatile anesthetics, Ca
channel block-ers, and ketamine, also may inhibit Na channels. Tetrodotoxin is
a poison that specifically binds Na channels but at a site on the exterior of
the plasma membrane. Human studies are under way with similar toxins to
determine whether they might provide effective, prolonged analgesia after local
infiltration.
Sensitivity of nerve fibers to
inhibition by local anesthetics is determined by axonal diameter,myelination,
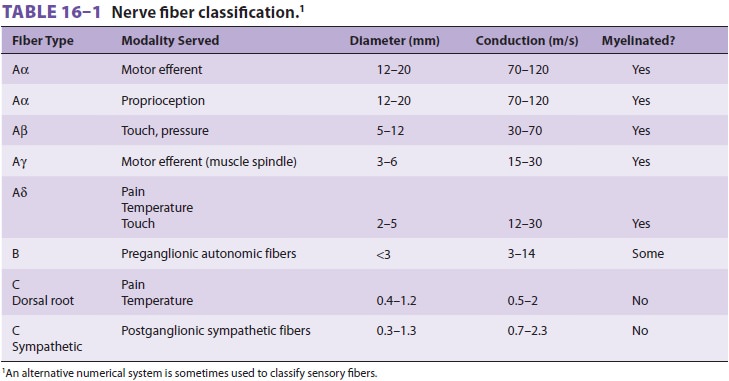
and other anatomic and physiological factors. Table 16–1 lists the most
commonly used classification for nerve fibers. In comparing nerve fibers of the
same type, small diameter increases sensitivity to local anesthetics. Thus,
larger, faster Aα fibers are less sensitive to local anesthetics than
smaller, slower-conducting Aδ fibers, and larger unmyelinated fibers
are less sensitive than smaller unmyelinated fibers. On the other hand, small
unmyelinated C fibers are relatively resistant to inhibition by local
anesthetics as compared with larger myelinated fibers. In spinal nerves local
anes-thetic inhibition (and conduction failure) generally follows the sequence
autonomic > sensory > motor, but at steady state if sensory
anesthesia is present all fibers are inhibited.
Related Topics