Chapter: Clinical Anesthesiology: Clinical Pharmacology: Local Anesthetics
Local Anesthetic: Effects on Organ Systems
Effects on Organ Systems
Because inhibition of voltage-gated Na
channels from circulating local anesthetics might affect action potentials in
neurons throughout the body as well as impulse generation and conduction in the
heart, it is not surprising that local anesthetics in high cir-culating
concentrations could have the propensity for systemic toxicity. Although organ
system effects are discussed for these drugs as a group, individual drugs
differ.
Potency at most toxic side effects
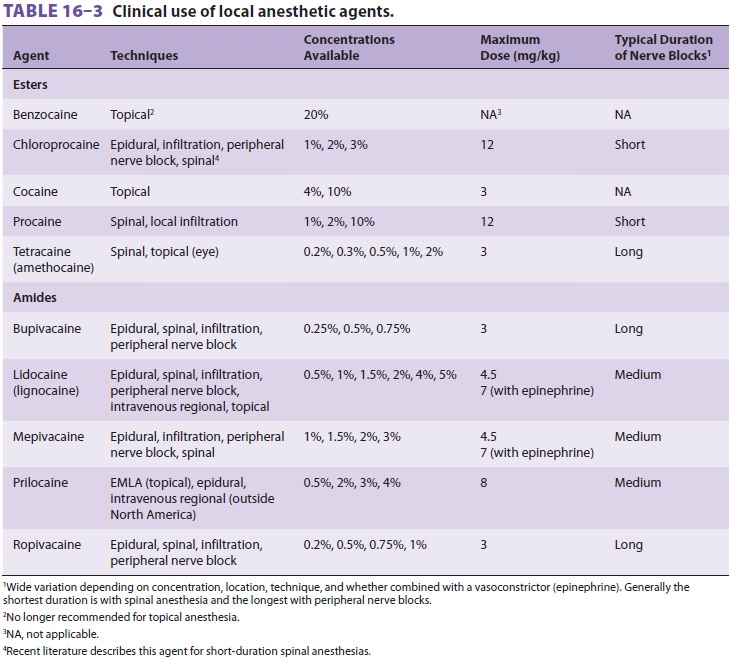
correlates with potency at nerve blocks. Maximum safe doses are listed in Table 16–3,
but it must be recognized that the maximum safe dose depends on the patient,
the specific nerve block, the rate of injection, and a long list of other
factors. In other words, tables of pur-ported maximal safe doses are nearly
nonsensical. Mixtures of local anesthetics should be considered to have
additive toxic effects; therefore, a solution containing 50% of the toxic dose
of lidocaine and 50% of the toxic dose of bupivacaine if injected by accident
intravenously will produce toxic effects.
A. Neurological
The central nervous system is vulnerable
to local anesthetic toxicity and is the site of premonitory signs of rising
blood concentrations in awake patients. Early symptoms include circumoral numbness,
tongue paresthesia, dizziness, tinnitus, and blurred vision. Excitatory signs
include restless-ness, agitation, nervousness, garrulousness, and a feeling of
“impending doom.” Muscle twitching her-alds the onset of tonic–clonic seizures.
Still higher blood concentrations may produce central nervous system depression
(eg, coma and respiratory arrest). The excitatory reactions are thought to be
the result of selective blockade of inhibitory pathways. Potent, highly
lipid-soluble local anesthetics produce sei-zures at lower blood concentrations
than less potent agents. Benzodiazepines and hyperventilation raise the
threshold of local anesthetic-induced seizures. Both respiratory and metabolic
acidosis reduce the seizure threshold. Propofol (0.5–2 mg/kg) quickly and
reliably terminates seizure activity (as do com-parable doses of
benzodiazepines or barbiturates). Maintaining a clear airway with adequate
ventilation and oxygenation is of key importance.
Infused local anesthetics have a variety of actions. Systemically administered local anesthetics such as lidocaine (1.5 mg/kg) can decrease cerebral blood flow and attenuate the rise in intracranial pressure that may accompany intubation in patients with decreased intracranial compliance. Infusions of lidocaine and procaine have been used to sup-plement general anesthetic techniques, as they are capable of reducing the MAC of volatile anesthetics by up to 40%. Infusions of lidocaine inhibit inflam-mation and reduce postoperative pain. Infused lido-caine reduces postoperative opioid requirements sufficiently to reduce length of stay after colorectal or open prostate surgery.Cocaine stimulates the central nervous system and at moderate doses usually causes a sense of euphoria. An overdose is heralded by restlessness, emesis, tremors, convulsions, arrhythmias, respira-tory failure, and cardiac arrest.
Local anesthetics temporarily inhibit
neuro-nal function. In the past, unintentional injection of large volumes of
chloroprocaine into the sub-arachnoid space (during attempts at epidural
anesthesia), produced total spinal anesthesia and marked hypotension, and
caused prolongedneurological deficits. The cause of this neural tox-icity may
be direct neurotoxicity or a combination of the low pH of chloroprocaine and a
preserva-tive, sodium bisulfite. The latter has been replaced in some
formulations by an antioxidant, a deriva-tive of disodium
ethylenediaminetetraacetic acid (EDTA). Chloroprocaine has also been
occasion-ally associated with severe back pain following epidural
administration. The etiology is unclear. Chloroprocaine is available in a
preservative-free formulation, which has been used in recent studies safely and
successfully for short duration, outpatient spinal anesthetics.
Administration of 5% lidocaine has been
asso-ciated with neurotoxicity (cauda equina syndrome) following infusion
through small-bore catheters used in continuous spinal anesthesia. This may be
due to pooling of drug around the cauda equina, resulting in high
concentrations and permanent neuronal damage. Animal data suggest that the
extent of histological evidence of neurotoxicity fol-lowing repeat intrathecal
injection is lidocaine = tetracaine > bupivacaine > ropivacaine.
Transient neurological symptoms, which
con-sist of dysesthesia, burning pain, and aching in the lower extremities and
buttocks, have been reported following spinal anesthesia with a variety of
local anesthetic agents, most commonly after use of lidocaine for outpatient
spinal anesthesia in men undergoing surgery in the lithotomy position. These
symptoms have been attributed to radicular irrita-tion and typically resolve
within 1–4 weeks. Many clinicians have substituted 2-chloroprocaine,
mepi-vacaine, or small doses of bupivacaine for lidocaine in spinal anesthesia
in the hope of avoiding these transient symptoms.
B. Respiratory
Lidocaine depresses hypoxic drive (the
ventila-tory response to low PaO2). Apnea can result from phrenic and
intercostal nerve paralysis or depres-sion of the medullary respiratory center
following direct exposure to local anesthetic agents (as may occur after
retrobulbar blocks;). Apnea after administration of a “high” spinal or epidural
anesthetic is nearly always the result of hypotension, rather than phrenic block.
Local anesthetics relax bronchial smooth muscle. Intravenous lidocaine (1.5
mg/kg) may be effective in blocking the reflex bronchoconstriction some-times
associated with intubation. Lidocaine (or any other inhaled agent) administered
as an aerosol can lead to bronchospasm in some patients with reac-tive airway
disease.
C. Cardiovascular
All local anesthetics depress myocardial
automa-ticity (spontaneous phase IV depolarization). Myocardial contractility
and conduction velocity are also depressed at higher concentrations. These
effects result from direct cardiac muscle membrane changes (ie, cardiac Na
channel blockade) and in intact organisms from inhibition of the autonomic
nervous system. All local anesthetics except cocaine produce smooth muscle relaxation
at higher con-centrations, which may cause some degree of arte-riolar
vasodilation. At low concentrations all local anesthetics inhibit nitric oxide,
causing vasocon-striction. At increased blood concentrations the combination of
arrhythmias, heart block, depres-sion of ventricular contractility, and
hypotension may culminate in cardiac arrest. Major car-diovascular toxicity
usually requires aboutthree times the local anesthetic concentration in blood
as that required to produce seizures. Cardiac arrhythmias or circulatory
collapse are the usual presenting signs of local anesthetic overdose dur-ing
general anesthesia. Particularly in awake sub-jects, signs of transient
cardiovascular stimulation (tachycardia and hypertension) may occur with
central nervous system excitation at local anes-thetic concentrations producing
central nervous system toxic side effects.
Intravenous amiodarone provides
effective treatment for some forms of ventricular arrhyth-mias. Myocardial
contractility and arterial blood pressure are generally unaffected by the usual
intravenous doses. The hypertension associated with laryngoscopy and intubation
is attenuated in some patients by intravenous administration of lidocaine (1.5
mg/kg) 1–3 min prior to instrumen-tation. On the other hand, overdoses of
lidocaine can lead to marked left ventricular contractile
dysfunction.Unintentional intravascular injection of bupi-vacaine during
regional anesthesia may produce severe cardiovascular toxicity, including left
ventricular depression, atrioventricular heart block, and life-threatening
arrhythmias such as ventricular tachycardia and fibrillation. Pregnancy,
hypoxemia, and respiratory acidosis are predisposing risk fac-tors. Young
children may also be at increased risk of toxicity. Multiple studies have
demonstrated that bupivacaine is associated with more pronounced changes in
conduction and a greater risk of terminal arrhythmias than comparable doses of
lidocaine. Mepivacaine, ropivacaine, and bupivacaine have chiral carbons and
therefore can exist in either of two optical isomers (enantiomers). The R(+) optical isomer
of bupivacaine blocks more avidly and dis-sociates more slowly from cardiac Na
channels than does the S(−) optical isomer. Resuscitation from
bupivacaine-induced cardiac toxicity is often diffi-cult and resistant to
standard resuscitation drugs. Recent reports suggest that bolus administration
of nutritional lipid solutions at 1.5 mL/kg can resusci-tate
bupivacaine-intoxicated patients who do not respond to standard therapy. Ropivacaine
shares many physicochemical properties with bupivacaine. Onset time and
duration of action are similar, but ropivacaine produces less motor block when
injected at the same volume and concentration as bupiva-caine (which may
reflect an overall lower potency as compared with bupivacaine). Ropivacaine
appears to have a greater therapeutic index than bupivacaine. This improved
safety profile likely reflects its formu-lation as a pure S(−) isomer—that
is, having no R(+) isomer—as opposed to racemic bupivacaine.
Levobupivacaine, the S(−) isomer of bupivacaine, which is no
longer available in the United States, was reported to have fewer
cardiovascular and cerebral side effects than the racemic mixture; studies
sug-gest its cardiovascular effects may approximate those of ropivacaine.
Cocaine’s cardiovascular reactions are
unlike those of any other local anesthetic. Adrenergic nerve terminals normally
reabsorb norepineph-rine after its release. Cocaine inhibits this reup-take,
thereby potentiating the effects of adrenergic stimulation. Cardiovascular
responses to cocaine include hypertension and ventricular ectopy. The latter
contraindicated its use in patients anesthetized with halothane. Cocaine-induced arrhythmias have been
successfully treated with adrenergic and Ca channel antagonists. Cocaine
produces vasocon-striction when applied topically and is a useful agent to
reduce pain and epistaxis related to nasal intuba-tion in awake patients.
D. Immunological
True hypersensitivity reactions to local
anes-thetic agents—as distinct from systemic toxicity caused by excessive
plasma concentration—are uncommon. Esters appear more likely to induce a true
allergic reaction (due to IgG or IgE antibodies) especially if they are
derivatives (eg, procaine or benzocaine) of p-aminobenzoic
acid, a known allergen. Commercial multidose preparations of amides often
contain methylparaben, which has a
chemical structure vaguely similar to that of PABA. As a consequence,
generations of anesthesiologists have speculated whether this preservative may
be responsible for most of the apparent allergic responses to amide agents.
E. Musculoskeletal
When directly injected into skeletal
muscle (eg, trigger-point injection treatment of myofas-cial pain), local
anesthetics are mildly myotoxic. Regeneration usually occurs 3–4 weeks after
local anesthetic injection into muscle. Concomitant steroid or epinephrine
injection worsens the myonecrosis.
F. Hematological
Lidocaine mildly depresses normal blood
coagula-tion (reduced thrombosis and decreased platelet aggregation) and
enhances fibrinolysis of whole blood as measured by thromboelastography. These
effects may underlie the reduced efficacy of an epi-dural autologous blood
patch shortly after local anesthetic administration and the lower incidence of
embolic events in patients receiving epidural anesthetics (in older studies of
patients not receiving prophylaxis against deep vein thrombosis).
Related Topics