Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Viral Genetics
Malignant Transformation - Viral Genetics
MALIGNANT TRANSFORMATION
A tumor is an abnormal growth of cells. Tumors are classified as benign or malignant, de-pending on whether they remain localized or have a tendency to invade or spread by metastasis. Therefore, malignant cells have at least two defects. They fail to respond to controlling signals that normally limit the growth of nonmalignant cells, and they fail to recognize their neighbors and remain in their proper location. When grown in tissue cul-ture in the laboratory, these tumor cells exhibit a series of properties that correlate with the uncontrolled growth potential associated with the tumor in the organism.
1. They have altered cell morphology.
2. They fail to grow in the organized patterns found for normal cells.
3. They grow to much higher cell densities than do normal cells under conditions of un-limited nutrients; therefore, they appear unable to enter the resting G0 state.
4. They have lower nutritional and serum requirements than normal cells.
5. They have the capacity to divide in suspension, whereas normal cells require an an-choring substrate and grow only on surfaces (eg, glass or plastic).
6. They are usually able to grow indefinitely in cell culture.
Many DNA animal viruses and some representatives of the retroviruses can con-vert normal cultured cells into cells that possess the properties listed above. This process is called malignant transformation. In addition to the listed properties, viral transformation usually, but not always, endows the cells with the capacity to form a tumor when introduced into the appropriate animal. Although the original use of the term transformationreferred to the changes occurring in cells grown in the labora-tory, current usage often includes the initial events in the animal that lead to the devel-opment of a tumor. In recent years, it has become increasingly clear that some but not all of these viruses also cause cancers in the host species from which they were isolated.
Transformation by DNA Animal Viruses
The oncogenic potential of animal DNA viruses is summarized in Table 7 – 1. All known DNA animal viruses, except parvoviruses, are capable of causing aberrant cell prolifera-tion under some conditions. For some viruses, transformation or tumor formation has been observed only in species other than the natural host. Apparently infections of cells from the natural host are so cytocidal that no survivors remain to be transformed. In addi-tion, some viruses have been implicated in human or animal tumors without any indica-tion that they can transform cells in culture.
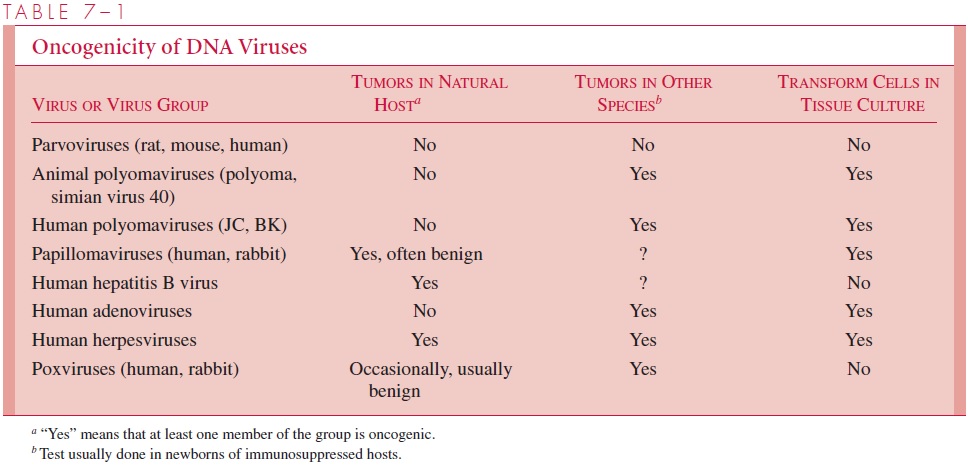
In nearly all cases that have been characterized, viral transformation is the result of the continual expression of one or more viral genes that are directly responsible for the loss of growth control. Two targets have been identified that appear to be critical for the transforming potential of these viruses. Adenoviruses, papilloma viruses, and simian virus 40 all code for either one or two proteins that interact with the tumor suppressor proteins known as p53 and Rb (for retinoblastoma protein) to block their normal function which is to exert a tight control over cell cycle progression. The end result is endless cell cycling and uncontrolled growth.
In many respects, transformation is analogous to lysogenic conversion and requires that the viral genes be incorporated into the cell as inheritable elements. Incorporation usually involves integration into the chromosome (eg, papovaviruses, adenoviruses, and retroviruses), although the DNAs of some papillomaviruses and some herpesviruses are found in transformed cells as extrachromosomal plasmids. Unlike some of the temperate bacteriophages that code for the enzymes necessary for integration, papovaviruses and adenoviruses integrate by nonhomologous recombination using enzymes present in the host cell. The recombination event is therefore nonspecific, both with respect to the viral DNA and with respect to the chromosomal locus at which insertion occurs. It follows that for transformation to be successful, the insertional recombination must not disrupt a viral gene required for transformation. In summary, two events appear to be necessary for viral transformation: a persistent association of viral genes with the cell and the expression of certain viral “transforming” proteins.
Transformation by Retroviruses
Two features of the replicative cycle of retroviruses are related to the oncogenic potential of this class of viruses. First, most retroviruses do not kill the host cell, but instead set up a permanent infection with continual virus production. Second, a DNA copy of the RNA genome is integrated into the host cell DNA by a virally encoded integrase (IN); however, unlike bacteriophage integration, a linear form of the viral DNA, rather than a circular form, is the substrate for integration. Furthermore, unlike , there does not appear to be a specific site in the cell DNA where integration occurs.
Retroviruses are known to transform cells by three different mechanisms. First, many animal retroviruses have acquired transforming genes calledoncogenes. More than 30 such oncogenes have now been found since the original oncogene was identified in Rous sar-coma virus (called v-src, where the v stands for viral). Because normal cells possess hom-ologs of these genes called protooncogenes (eg, c-src, where c stands for cellular), it is generally thought that viral oncogenes originated from host DNA. It is possible they were picked up by “copy choice” recombination involving packaged cellular mRNAs as previ-ously described. Because these transforming viruses carry cellular genes, they are some-times referred to astransducing retroviruses. Most of the viral oncogenes have suffered one or more mutations that make them different from the cellular protooncogenes. These changes presumably alter the protein products so that they cause transformation. Although the mechanisms of oncogenesis are not completely understood, it appears that transforma-tion results from inappropriate production of an abnormal protein that interferes with nor-mal signaling processes within the cell. Uncontrolled cell proliferation is the result. Because tumor formation by retroviruses carrying an oncogene is efficient and rapid, these viruses are often referred to as acute transforming viruses. Although common in some animal species, this mechanism has not yet been recognized as a cause of any human cancers.
The second mechanism is called insertional mutagenesis and is not dependent on continued production of a viral gene product. Instead, the presence of the viral promoter or enhancer is sufficient to cause the inappropriate expression of a cellular gene residing in the immediate vicinity of the integrated provirus. This mechanism was first recognized in avian B-cell lymphomas caused by an avian leukosis virus, a disease characterized by a very long latent period. Tumor cells from different individuals were found to have a copy of the provirus integrated at the same place in the cellular DNA. The site of the provirus insertion was found to be next to a cellular protooncogene called c-myc. The myc gene had previously been identified as a viral oncogene called v-myc. In this case, transforma-tion occurs not because the c-myc gene is altered by mutation but because the viral pro-moter adjacent to the gene turns on its expression continuously and the gene product is overproduced. The disease has a long latent period; because, although the birds are viremic from early life, the probability of an integration occurring next to the c-myc gene is very low. Once such an integration event does occur, however, cell proliferation is rapid and a tumor develops. No human tumors are known for certain to result from insertional mutagenesis caused by a retrovirus; however, human cancers are known where a chromo-some translocation has placed an active cellular promoter next to a cellular protoonco-gene (Burkitt’s lymphoma and chronic myelogenous leukemia).
The third mechanism was revealed by the discovery of the first human retrovirus. The virus, human T-cell lymphotropic virus type 1 (HTLV-1), is the causative agent of adult T-cell leukemia. HTLV-1 sequences are found integrated in the DNA of the leukemic cells and all the tumor cells from a particular individual have the proviral DNA in the same location. This observation indicates that the tumor is a clone derived from a single cell; however, the sites of integration in tumors from different individuals are different. Thus, HTLV-1 does not cause malignancy by promoter insertion near a particular cellular gene. Instead, the virus has a gene called tax that codes for a protein that acts in trans (ie, on other genes in the same cell) to not only promote maximal transcription of the proviral DNA, but also to transcriptionally activate an array of cellular genes. The resulting cellu-lar proteins cooperate to cause uncontrolled cell proliferation. The tax gene is therefore different from the oncogenes of the acute transforming retroviruses in that it is a viral gene rather than a gene derived from a cellular protooncogene. HTLV-1 is commonly de-scribed as a transactivatingretrovirus.
Related Topics