Chapter: Physics : Magnetic Materials
Magnetic Materials
MAGNETIC MATERIALS
1 Introduction
2 Terms and definitions
3 Origin of Magnetic moment
3.1 Orbital Magnetic moment
3.2 Bohr magneton
4 Different types of magnetic materials
4.1 Diamagnetic materials
4.2 Paramagnetic materials
4.3 Ferromagnetic materials
4.4 Anti Ferro magnetic materials
4.5 Ferri magnetic materials
5 Ferromagnetism
6 Domain theory of ferromagnetism
6.1 Domain magnetization
6.2 Energies involved in the domain growth
6.3 Explanation of Hysteresis based on domain theory
7 Soft and Hard magnetic materials
8 Energy product
9 Ferri magnetic materials – Ferrites
9.1. Structure of ferrites
9.2. Preparation
9.3. Properties
9.4. Advantages
9.5. Disadvantages
9.6. Applications
1. INTRODUCTION
Any materials that can be magnetized by an applied by an applied external magnetic field is called a magnetic materials. Magnetic materials can be easily magnetized because they have permanent or induced magnetic moment in the presence of applied magnetic field. Magnetism arise from the magnetic moment or magnetic dipole of the magnetic materials. Among the different eleven types of magnetic materials, only five magnetic materials are the most important for the practical application. They are:
§ Diamagnetic materials.
§ Paramagnetic materials.
§ Ferromagnetic materials.
§ Antiferromagnetic materials.
§ Ferrimagnetic materials or ferrites.
2 TERMS AND DEFINITIONS
Magnetic flux (φ)
Total number of magnetic lines of force passing through a surface is known as magnetic flux. It is represented by the symbol ‘φ’ and its unit
Magnetic flux density (or) Magnetic induction (B)
Magnetic flux density at any point in a m passing normally through unit area of cross section (A) at that point. It is denoted by the symbol B and
its unit is weber / metre2 or tesla.
B = [φ / A]
Intensity of magnetization (I)
The term magnetization means the process of converting non-magnetic material on magnetic material.
When some amount of external magnetic field is applied to the metals such as iron, steel and alloys etc., they are magnetized to different degrees. The intensity of magnetisation (I) is the measure of the magnetisation of a magnetized specimen. It is defined as the magnetic moment per unit volume.
I = M / V weber / metre2
Magnetic field intensity (or) strength (H)
Magnetic field intensity at any point in a magnetic field is the force experienced by unit north pole placed at that point.
It is denoted by H and its unit is Newton per weber or ampere turns per metre (A/m).
Magnetic permeability (μ)
Magnetic permeability of a substance measure the degree to which the magnetic field can penetrate through the substance.
It is found that magnetic flux density (B) is directly proportional to the magnetic field strength (H)
B α H
B = μ H
Where is a constant of proportionality and it is known as permeability or absolute permeability of the medium.
μ = B / H
Hence, the permeability of a substance is the ratio of the magnetic flux density (B) inside the substance to the magnetic field intensity (H).
Absolute permeability
Absolute permeability of a medium or material is defined as the product of permeability of free space (μ 0) and the relative permeability. of the medium (μ r)
μ = μ0x μr
Relative permeability of medium (μ r )
Relative permeability of a medium is defined as the ratio between absolute permeability of a medium to the permeability of a free space
μr = μ / μ0
Magnetic susceptibility (χ)
Magnetic susceptibility (χ) of a specimen magnetized in a magnetic field.
It is the ratio of intensity of magnetisation (I) induced in it to the magnetizing field (H).
χ = I /H
Retentivity (or) Remanence
When the external magnetic field is applied to a magnetic material is removed, the magnetic material will not loss its magnetic property immediately. There exits some residual intensity of magnetization in the specimen even when the magnetic field is cut off. This is called residual magnetism (or) retentivity.
Coercivity
The residual magnetism can be completely removed from the material by applying a reverse magnetic field. Hence coercivity of the magnetic material is the strength of reverse magnetic field (-Hc) which is used to completely demagnetize the material.
3 ORIGIN OF MAGNETIC MOMENT AND BOHR MAGNETON
3.1 Origin of magnetic moment
Any matter is basically made up of atoms. The property of magnetism exhibited by certain materials with the magnetic property of its constituent atoms. We know that electrons in an atom revolve around the nucleus in different orbits.
Basically there are three contributions for the magnetic dipole moment of an atom.
The orbital motions of electrons (the motion of electrons in the closed orbits around the nucleus) are called orbital magnetic moment.
Spin motion of the electrons (due to electron spin angular momentum) is called spin magnetic moment.
The contribution from the nuclear spin (due to nuclear spin angular momentum) is nearly 10 3 times smaller than that of electron spin; it is not taken into consideration.
3.2 Bohr Magneton
The magnetic moment contributed by an electron with angular momentum quantum number n = 1 is known as Bohr Magneton.
4 DIFFERENT TYPES OF MAGNETIC MATERIALS
4.1. DIAMAGNETIC MATERIALS
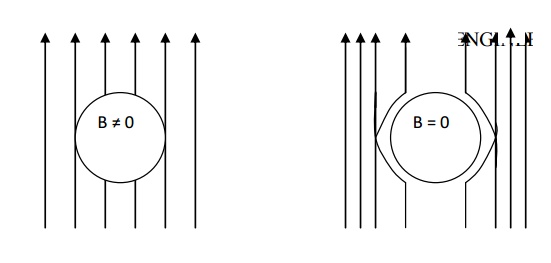
Diamagnetism is exhibited by all the materials. The atoms in the diamagnetic materials do not possess permanent magnetic moment.
However, when a material is placed in a magnetic field, the electrons in the atomic orbits tend to counteract the external magnetic field and the atoms acquire an induced magnetic moment.
As a result, the material becomes magnetized. The direction of the induced dipole moment is opposite to that of externally applied magnetic field. Due to this effect, the material gets very weakly repelled, in the magnetic field. This phenomenon is known as diamagnetism.
When a magnetic field Ho is applied in the direction shown in fig., the atoms acquire an induced magnetic moment in the opposite direction to that of the field.
The strength of the induced magnetic moment is proportional to the applied field and hence magnetization of the material varies directly with the strength of the magnetic field.
The induced dipoles and magnetization vanish as soon as the applied field is removed.
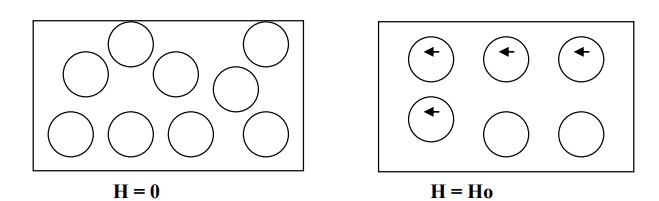
Properties of diamagnetic material
Diamagnetic magnetic material repels the magnetic lines of force. The behaviour of diamagnetic material in the presence of magnetic field.
There is no permanent dipole moment. Therefore, the magnetic effects are very small.
The magnetic susceptibility is negative and it is independent of temperature and applied magnetic field strength.
4.2. PARAMAGNETIC MATERIALS
In certain materials, each atom or molecule possesses a net permanent magnetic moment (due to orbital and spin magnetic moment) even in the absence of an external magnetic field.
The magnetic moments are randomly oriented in the absence of external magnetic field. Therefore the net magnetic moment is zero, and hence the magnetization of the material is zero.
But, when an external magnetic field is applied, the magnetic dipoles tend to align themselves in the direction of the magnetic field and the material becomes magnetized. As shown in fig. This effect is known as paramagnetism.
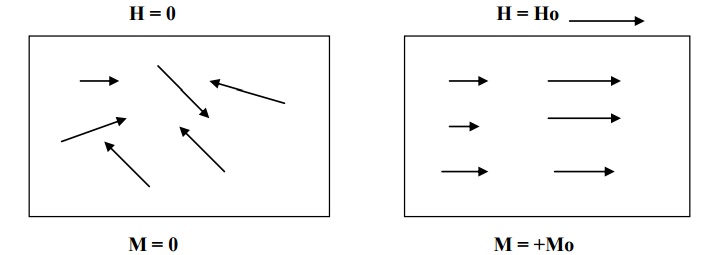
Thermal agitation disturbs the alignment of the magnetic moments. With an increase in temperature, the increase in thermal agitation tends to randomize the dipole direction thus leading to a decrease in magnetization.
This indicates that the paramagnetic susceptibility decreases with increases in temperature. It is noted that the paramagnetic susceptibility varies inversely with temperature.
χ α 1 / T
χ=C / T
This is known as Curie law of paramagnetism and C is a constant called Curie constant
Properties of paramagnetic materials
Paramagnetic materials attract magnetic lines of force.
They possess permanent dipole moment.
The susceptibility is positive and depend on temperature is given by
χ = –Cθ / T
The spin alignment is shown in fig.
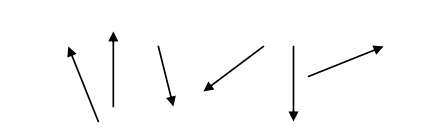
Example- Manganous sulphate, ferric oxide, ferrous sulphate, nickel sulphate, etc.
4.3. FERROMAGNETIC MATERIALS
Certain materials like iron, cobalt, nickel and certain alloys exhibit high degree of magnetization. These materials show spontaneous magnetization. (i.e) they have small amount of magnetization even in the absence of external magnetic field.
This indicates that there is strong internal field within the material which makes atomic magnetic moments with each other. This phenomenon is known as ferromagnetism.
Properties of ferromagnetic materials:
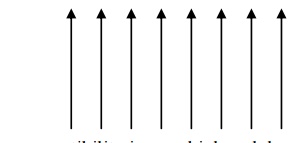
All the dipoles are aligned parallel to each other due to the magnetic interaction between the two dipoles.
They have permanent dipole moment. They are strongly attracted by the magnetic field.
They exhibit magnetization even in the absence of magnetic field. This property of ferromagnetic material is called as spontaneous magnetization.
They exhibit hysteresis curve.
On heating, they lose their magnetization slowly. The dipole alignment is shown in fig.
The
The susceptibility is very high and depends on the temperature. It is given by
χ = C /T –θ
[ for T>θ; paramagnetic behaviour;
for T<θ; ferromagnetic behaviour]
Where C is the Curie constant and θ is the paramagnetic Curie temperature.
4.4.ANTIFERROMAGNETIC MATERIALS
Antiferromagnetic materials are magnetic materials which exhibit a small positive susceptibility of the order of 10 -3 to 10-5.
In antiferromagnetic materials, the susceptibility increases with increasing temperature and it reaches maximum at a certain temperature called Neel Temperature, TN.
With further increase in temperature, the material reaches the paramagnetic state. The material is antiferromagnetic below TN.
Properties of antiferromagnetic materials
The electron spin of neighboring atoms are aligned antiparallel. (i.e) the spin alignment is antiparallel.
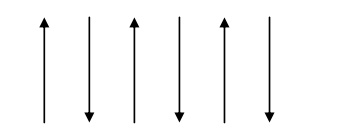
Antiferromagnetic susceptibility is mainly depends on temperature.
The susceptibility of the antiferromagneitc material is small and positive. It is given by
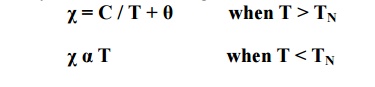
The susceptibility initially increases slightly with the temperature and beyond Neel temperature, the susceptibility decreases with temperature.
4.5.FERRIMAGNETIC MATERIALS
Properties of ferrites
Ferrites have net magnetic moment.
Above Curie temperature, it becomes paramagnetic, while it behaves ferromagnetic material blow Curie temperature.
The susceptibility of ferrite is very large and positive. It is temperature dependent and is given by
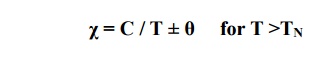
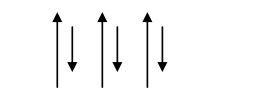
Spin alignment is antiparallel of different magnitudes as shown fig.
Mechanically it has pure iron character.
They have high permeability and resistivity.
They have low eddy current losses and low hysteresis losses.
5 FERROMAGNETISM
The materials which have finite value of magnetization even if the external magnetic field is absent are called ferromagnetic materials. This phenomenon is called ferromagnetism. The ferromagnetic materials exhibit high degree of magnetization.
Explanation
In a ferromagnetic material, the magnetic interactions between any two dipoles align themselves parallel to each other. Ferromagnetism arises due to the special form of interaction called exchange coupling between adjacent atoms. This exchange coupling is favourable for spin alighnment and they coupling their magnetic moments together in rigid parallelism.
A ferromagnetic materials exibits ferromagnetic property below a particular temperature called ferromagnetic. Curie temperature (fƟ). Above fƟ they behaves as paramagnetic material.
6. DOMAIN THEORY OF FERROMAGNETISM
We can observe that ferromagnetic materials such as iron does not have magnetization unless they have been previously placed in an external magnetic field. But according to Weiss theory, the molecular magnets in the ferromagnetic material are said to be aligned in such way that, they exhibit magnetization even in the absence of external magnetic field. This is called spontaneous magnetization. (i.e) it should have some internal magnetization due to quantum exchange energy.
According to Weiss hypothesis, a single crystal of ferromagnetic material is divided into large number of small regions called domains. These domains have spontaneous magnetization due to the parallel alignment of spin magnetic moments in each atom. But the direction of spontaneous magnetization varies from domain to domain and is oriented in such way that the net magnetization of the specimen is zero
The boundaries separating the domains are called domain walls. These domain walls are analogous to the grain boundaries in a polycrystalline material.
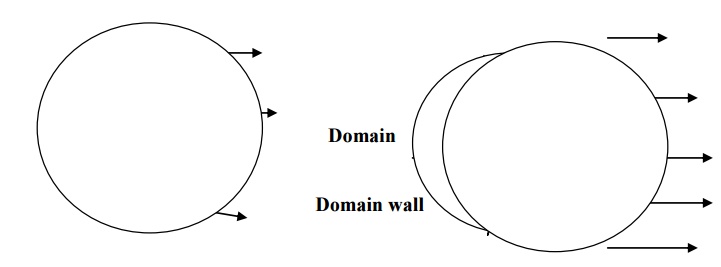
6.1. DOMAIN MAGNETIZATION
Now when the magnetic field is applied, then the magnetization occurs in the specimen by two ways
By moment of domain walls
By rotation of domain walls
moment of domain walls
The moment of domain walls takes place in weak magnetic fields. Due to this weak field applied to the specimen the magnetic moment increases and hence the boundary of domains displaced, so that the volume of the domains changes as shown in fig.
By rotation of domain walls
The rotation of domain walls takes place in strong magnetic fields. When the external field is high then the magnetization changes by means of rotation of the direction of magnetization towards the direction of the applied field as shown fig.
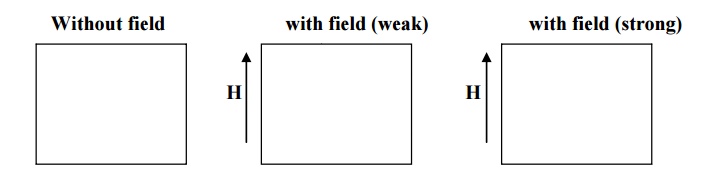
6.2. ENERGIES INVOLVED IN DOMAIN GROWTH
To study the domain structure clearly, we must know four types of energy involved in the process of domain growth. They are
Exchange energy
Anisotropy energy
Domain wall energy
Magneto- strictive energy
Exchange energy (or) magnetic field energy (or) magneto-static energy
The interacting energy which makes the adjacent dipoles to align themselves is known exchange energy (or) magnetic field energy. The exchange energy has established a single domain in a specimen of ferromagnetic and it is shown in fig.
It is the energy required in assembling the atomic magnets in a single domain and this work done is
stored as potential energy.
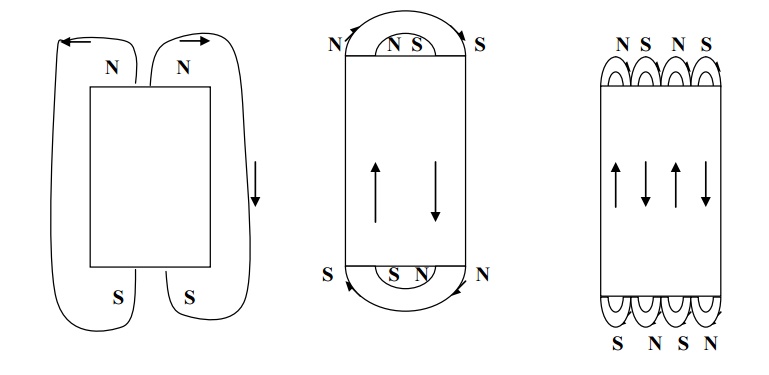
Anisotropy energy
In ferromagnetic crystals there are two direction of magnetization.
Easy direction
Hard direction
In easy direction of magnetization, weak field can be applied and in hard direction of magnetization, strong field should be applied. For producing the same saturation magnetization along both hard and easy direction, strong fields are required in the hard direction than the easy direction.
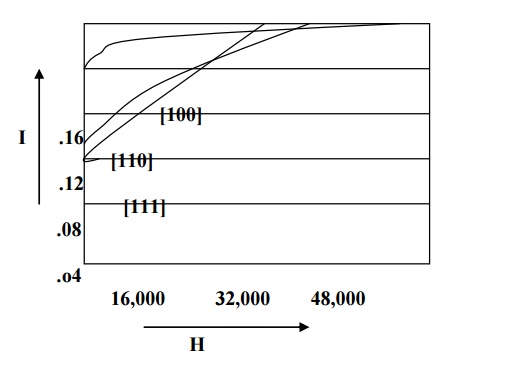
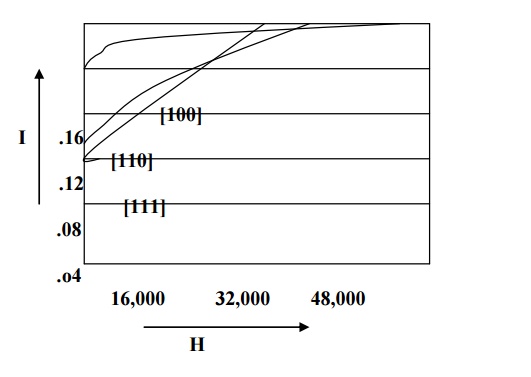
For example in iron easy direction is [100], medium direction is [110] and the hard direction is [111] and it is shown in fig. From the fig we can see that very strong field is required to produce magnetic saturation in hard direction [111] compared to the easy direction [100].
Therefore the excess of energy required to magnetize the specimen along hard direction over that required to magnetize the specimen along easy direction is called crystalline anisotropy energy.
Domain wall energy (or) Bloch wall energy
Domain wall is a transition layer which separates the adjacent domains, magnetized in different directions. The energy of domain wall is due to both exchange energy and anisotropy energy.
Based on the spin alignment, two types of domain walls may arise,
namely Thick wall
Thin wall
(i) Thick wall
When the spin at the boundary are misaligned if the direction of the spin changes gradually as shown in fig, it leads to a thick domain wall. Here the misalignments of spins are associated with exchange energy.
(ii) Thin wall
When the spin at the boundaries changes abruptly, then the anisotropy energy becomes very less. Since the anisotropy energy is directly proportional to the thickness if the wall, this leads to a thin Bloch wall.
Magetostrictive energy
When the domains are magnetized in different directions, they will either expand (or) shrink.
Therefore there exits a deformations (i.e) change in dimension of the material, when it is magnetized.
This phenomenon is known as magnetosriction and the energy produced in this effect is known as
magnetostriction energy.
The deformation is different along different crystal directions and the change dimension
depends upon the nature of the material.
6.3. EXPLANATION OF HYSTERESIS BASED ON DOMAIN THEORY
Hysteresis
When a ferromagnetic material is made to undergo through a cycle of magnetization, the variation of magnetic induction (B) with respect to applied field (H) can be represented by a closed hysteresis loop (or) curve. (i.e) it refers to the lagging of magnetization behind the magnetizing field.
If magnetizing field (H) is applied to a ferromagnetic material and if H is increases to Hmax the material acquires magnetism. So the magnetic induction also increases, represented by oa in the fig.
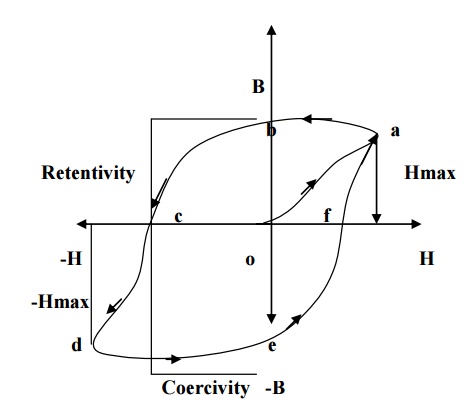
Now if the magnetic field is decreased from Hmax to zero, the magnetic induction will not fall rabidly to zero, but falls to ‘b’ rather than removed, the material still acquire some magnetic induction (ob) which is so called residual magnetism
or retntivity.
Now, to remove the residual magnetism, the magnetic field strength is reversed and increased to –Hmax represented as ‘oc’ so called coercivi-H)is
reduced to zero and the corresponding curve ‘de’ is obtai curve ‘efa’ is obtained.
We know when the ferromagnetic material is subjected to external field, there is an increase in the value of the magnetic moment due to two process.
The moment of domain walls
Rotation of domain walls
When small external field is applied, the domains walls displaced slightly in the easy direction of magnetization. This gives rise to small magnetization corresponding to the initial portion of the hysteresis curve (OA) as shown in fig.
Now of the field is removed, then the domains returns to the original state, and is known as reversible domains.
When the field is increased, large numbers of domains contribute to the magnetization and thus the magnetization increases rabidly with H.
Now, even when the field is removed, because of the displacement of domain wall to a very large distance, the domain boundaries do not come back to their original position. This process is indicating as AB in fig and these domains are called irreversible domains.
Now, when the field is further increased, the domains starts rotating along the field direction and the anisotropic energy stored in the hard direction, represented as BC in the fig.
Thus the specimen is said to attain the maximum magnetization. At this position, even when the field is removed the material posses maximum magnetization, called residual magnetism or retntivity, represented by OD in fig.
Actually after the removal of external field, the specimen will try to attain the original configuration by the moment of domain wall. But this moment is stopped due to presence of impurities, lattice imperfections etc. therefore to overcome this; a large amount of reverse magnetic field is applied to the specimen. The amount of energy spend to reduce the magnetization to zero is called coercivity represented by OE in the fig.
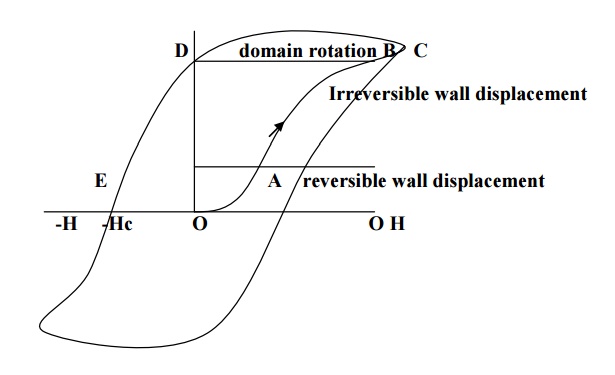
It is the loss of the energy in taking a ferromagnetic specimen through a complete cycle of magnetization and the area enclosed is called hysteresis loop.
7 SOFT AND HARD MAGNETIC MATERIALS
Depending upon the direction of magnetization by external field, and the area of hysteresis, magnetic can be classified into two types as,
TYPES OF MAGNETIC MATERIALS
Magnetic materials are classified onto two types.
Soft magnetic materials .
Hard magnetic material
Soft magnetic materials:
Materials which are easy to magnetize and demagnetize are called soft magnetic materials.
Example –pure iron, cast iron, carbon steel, silicon steel, mumetal.
Hard magnetic materials:
Materials which retain their magnetism and are difficult to demagnetize are called hard magnetic materials.
Example –tungsten steel, cobalt steel, alini, alnico, hypernic
Difference between soft and hard magnetic materials
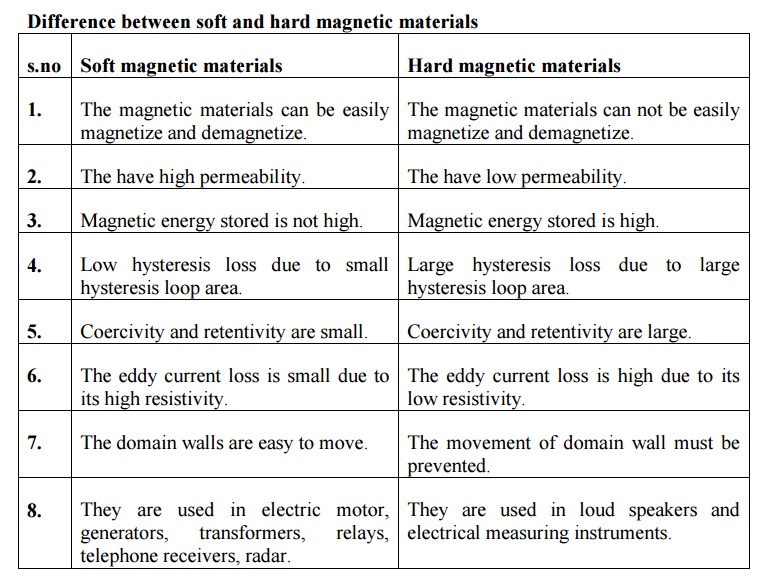
s.no Soft magnetic materials
1. The magnetic materials can be easily magnetize and demagnetize.
2. The have high permeability.
3. Magnetic energy stored is not high.
4. Low hysteresis loss due to small hysteresis loop area.
5. Coercivity and retentivity are small.
6. The eddy current loss is small due to its high resistivity.
7. The domain walls are easy to move.
8. They are used in electric motor, generators, transformers, relays, telephone receivers, radar.
Hard magnetic materials
1. The magnetic materials can not be easily magnetize and demagnetize.
2. The have low permeability.
3. Magnetic energy stored is high.
4. Large hysteresis loss due to large hysteresis loop area.
5. Coercivity and retentivity are large.
6. The eddy current loss is high due to its low resistivity.
7. The movement of domain wall must be prevented.
8. They are used in loud speakers and electrical measuring instruments.
8. ENERGY PRODUCT
Definition
The product of residual magnetic induction (Br) and coercivity(Hc) is called energy product or BH product. It is the important quantity to design powerful permanent magnets. It gives the maximum amount of energy stored in the specimen.
Explanation
The energy required to demagnetize a permanent magnet is given by the area of the hystersis loop between Br and Hc. The maximum value of this area Br Hc is called the energyproduct.
At C and D the energy product is zero because at C,H value is zero and D,B value is zero. The area occupied by the largest rectangle in demagnetizing curve gives the maximum (BH) value.
The energy product is large for permanent magnets. This value is very much useful to analyze whether the material can be used for magnetic recording.
9. FERRIMAGNETIC MATERIALS - FERRITES
Ferrites
Ferrites are components of iron oxide with oxides of other components.
The general chemical formula is X2+ Fe23+ O42- , where (X2+) is a divalent metal ion such as Fe2+, Mg2+, Ni2+, Co2+, Mn2+
9.1. STRUCTURE OF FERRITES
Generally there are two types of structures present in the ferrites. They are
Regular spinal
Inverse spinal
Regular spinal
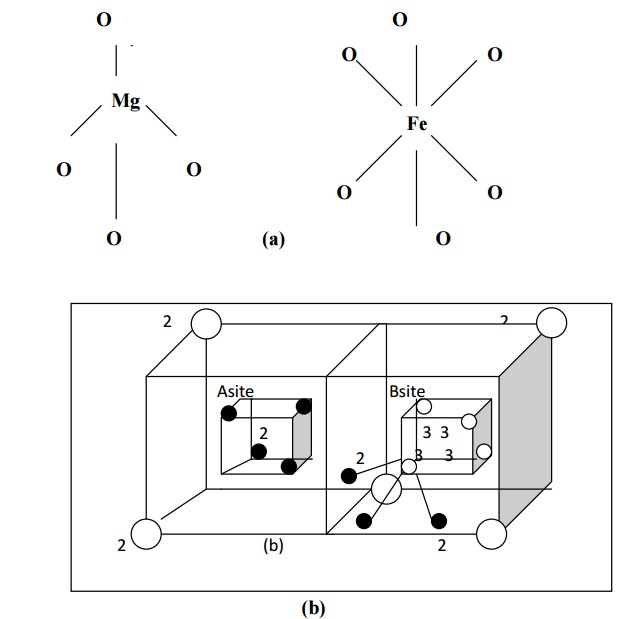
In regular spinal structure, each divalent metal ion is surrounded by four O2- ions in a tetrahedral fashion.
For example in Mg2+ Fe23+ O42-, the structure of Mg2+ is given in the fig (a) and it is called A site.
Each Fe3+ (trivalent metal ion) is surrounded by six O2- ions and forms an octahedral fashion as shown in fig (a). Totally there will be 16 such octahedral sites in the unit cell. This is indicated by B site.
Thus in regular spinal, each divalent metal ion (Mg2+) exits in tetrahedral form (A site) and each trivalent metal ion (Fe3+) exits in an octahedral form (B site). Hence the sites A and B combine together to form a regular spinal ferrite structure as shown in fig (b).
Inverse spinal
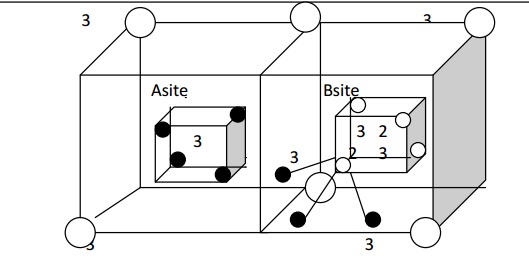
In this type, we consider the arrangement of ions of a single ferrous ferrite molecule Fe3+ [Fe2+ Fe3+] O42-. A Fe3+ ion (trivalent) occupies all A sites (tetrahedral) and half of the B sites (octahedral) also.
Thus the left out B sites will be occupied by the divalent (Fe2+). The inverse spinal structure is shown in fig (c).
9.2. PREPARATION
They have the general chemical composition A2+ Fe23+ O42- where A2+ represent a divalent metal ion like Zn2+, Mg2+, etc. Ferrities are prepared by sintering a mixture of various metallic oxides as follows.
1. Suitable of A2+ and Fe23+ O42- in proper proportions are mixed using water or kerosene.
2. The mixing is done in a blender for several hours. It is filtered.
3. The filtered material is dried in a hot oven and is then crushed.
4. Next mixture, is pre-sintered in a furnace at 9000c to 11000c for a period of three to fifteen hours, in an air atmosphere or nitrogen atmosphere.
5. The pre-sintered material is then ground into a fine powder and mixed with a binder such as paraffin wax and a solvent such as water.
6. The mixture is then pressed into the desired shapes by using dies.
7. The last step in the process is to place the ferrite in proper vessel in a furnace and heat it to about 1100to 14000c. The binder then evaporates. It is cooled in a controlled manner.
9.3PROPERTIES OF FERRITES
Ferrites have net magnetic moment.
Above Curie temperature, it becomes paramagnetic, while it behaves ferromagnetic material blow Curie temperature.
The susceptibility of ferrite is very large and positive. It is temperature dependent and is given by
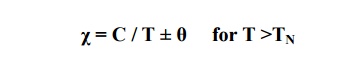
Spin alignment is antiparallel of different magnitudes as shown fig.
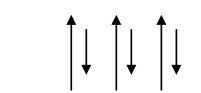
Mechanically it has pure iron character.
They have high permeability and resistivity.
They have low eddy current losses and low hysteresis losses.
9.4 AVANTAGES
1. Efficiency is high and cost is low.
2. They have low eddy current losses and low hysteresis losses.
3. Easy to manufacture with great uniformity.
4. They occupies low volume.
9.5. Disadvantages
1. The main disadvantage of bubble memory is the requirement of a high recording time for storing and retrieving the data than the charge coupled device (CCD).
2. It requires the interface circuits.
3. When compared with charge coupled device (CCD) memory the magnetic bubble memory has slow access speed.
9.6. Applications
They are used to produce ultrasonic waves by magnetostriction principle. Ferrites are used in audio and video transforms.
Ferrite rods are used in radio receivers to increase the sensitivity. They are also used in power limiting and harmonic generation. They are used in computers and data processing circuits.
Ferrites are used in paramagnetic amplifiers so that the input can be amplified with low noise figures.
Related Topics