Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Monoclonal Antibodies in Solid Organ Transplantation
Interleukin 2 Receptor Antagonists - Specific Agents Used In Solid Organ Transplant
Interleukin-2 Receptor Antagonists
IL-2 antagonists were the next monoclonal antibodies to be used and were specifically developed for use in solid organ transplantation. As previously mentioned, monoclonal antibody use and development in solid organ transplantation is rational. The IL-2 receptor was targeted for several reasons. IL-2, the ligand for the IL-2 receptor, is a highly conserved protein, with only a single gene locus on chromosome 4 (Church, 2003). Animal IL-2 knockout models have decreased lymphocyte function at 2 to 4 weeks of age and early mortality at 6 to 9 weeks of age (Chen and Harrison, 2002). These models also display significantly dimin-ished myelopoesis leading to severe anemia and global bone marrow failure (Chen and Harrison, 2002). This observation confirms the significant role that IL-2 and the IL-2 receptor complex play in immunity. The function and biological effect of IL-2 binding to the IL-2 receptor was first reported by Robb and Smith in 1981 (Robb and Smith in 1981). This in vitro study evaluated murine lymphocytes and found that the IL-2 receptor is only present on activated cells (CD4 þ and CD8 þ) (Church, 2003). Uchiyama and Waldmann (1981) reported one of the first monoclonal antibodies developed against acti-vated human T-cells. This compound displayed in vitro preferential activity against activated T-cells, including terminally mature T-cells, but did exhibit activity against B-cells or monocytes (Uchiyama and Waldmann, 1981). Later it was determined that this antibody actually bound to the alpha subunit of the activated T-cell receptor, CD25 (Church, 2003). The actual T-cell receptor is made up of three subunits, alpha, beta, and gamma. When the beta and gamma subunits combine they can only be stimulated by high concentrations of IL-2, however, in conjunction with the alpha subunit the receptor shows high affinity for IL-2 and can be stimulated at very low concentrations. The expression of IL-2 and the IL-2 receptor alpha region are highly regulated at the DNA transcriptionlevel, and is induced following T-cell activation (Shibuya et al., 1990). The alpha subunit is continu-ously expressed during allograft rejection, T-cell mediated autoimmune diseases, and malignancies (Church, 2003). The beta and gamma subunit, how-ever, has constitutive expression, resulting in low levels of expression in resting T-lymphocytes (Vincenti et al., 1997, 1998). There is no constitutive expression of IL-2 or the alpha receptor subunit (Shibuya et al., 1990; Noguchi et al., 1993). Both, the beta and gamma subunits, have similar molecular structures and are members of the cytokine receptor superfamily, but are structurally dissimilar to the alpha subunit (Noguchi et al., 1993). Therefore the alpha subunit (CD25) became a rational target for monoclonal development since it is only expressed on activated T-cells. Blockade of the CD25 receptor was to halt the activity of IL-2 thereby decreasing proliferation and clonal expansion of T-cells when activated by foreign donor antigens.
Daclizumab
In 1997, daclizumab became the first anti-CD25 monoclonal antibody approved for use in the preven-tion of allograft rejection in kidney transplant reci-pients, when combined with cyclosporine and corticosteroids. Daclizumab was also the first “huma-nized” monoclonal antibody approved in the United States for human administration (Tsurushita and Kumar, 2005). The daclizumab molecule is a human-ized IgG1 adapted from a mouse antibody against the alpha portion of the IL-2 receptor (Uchiyama and Waldmann, 1981). Daclizumab was developed as an alternative to the initial mouse antibody developed against the IL-2 receptor. The mouse antibody lead to the development of HAMA and inability to adminis-ter subsequent doses. Although daclizumab bound with one-third the affinity for the T-cell receptor site when compared to the original mouse molecule, it was still able to exhibit a high binding capacity (Ka ¼ 3 109M-1) (Tsurushita and Kumar, 2005; Queenet al., 1988). A daclizumab serum concentration of 1mg/mL is required for 50% inhibition of antigen induced T-cell proliferation (Junghans et al., 1990) Early, phase I clinical trials in kidney transplant recipients, who received corticosteroids in combina-tion with cyclosporine and azathioprine, used five doses of daclizumab (Vincenti et al., 1997). Pharmacokinetic studies revealed a mean serum half-life of 11.4 days, a steady-state volume of distribution of 5 L, and displayed weight dependent elimination. There was no change in the number of circulating CD3 positive cells following administra-tion. Five doses of 1 mg per kg body weight given every other week were required to produce the serumconcentrations needed to achieve 90% inhibition of T-cell proliferation for 12 weeks. One patient did develop neutralizing antibodies against the daclizi-mab molecule after receiving weekly doses for 2 weeks. Saturation of the IL-2 receptor did not change. Intravenous doses were well tolerated with no infusion-related reactions. No infection or malig-nancies were reported up to 1 year following daclizumab administration. The authors concluded that daclizumab stayed within the intravascular space and doses should be based on patient weight at the time of transplant (Vincenti et al., 1997). Subsequent pre-marketing clinical trials confirmed these results and dosing schematic and were able to show that daclizimab administration reduced the incidence of acute rejection by 13% in low risk kidney transplant recipients (Vincenti et al., 1998). Following daclizu-mab’s approval several trials have been conducted using various dosing regimens and immunosuppres-sion combinations within various solid organ recipi-ents. The exact number of doses or duration of therapy for each organ transplant recipient to achieve optimal therapy is currently unknown.
Basiliximab
Basiliximab was developed as a more potent anti-IL-2 receptor antagonist when compared to daclizumab and may have several logistical advantages. Basiliximab, in combination with cyclosporine and corticosteroids, was approved for the prevention of acute allograft rejection in renal transplant recipients in May, 1998. Basiliximab is a murine/human (chimeric) monoclonal antibody directed against the alpha sub-unit of the IL-2 receptor on the surface of activated T-lymphocytes. The antibody is produced from geneti-cally engineered mouse myeloma cells. The variable region of the purified monoclonal antibody is com-prised of murine hypervariable region, RFT5, which selectively binds to the IL-2 receptor alpha region. The constant region is made up of human IgG1 and kappa light chains (Novartis Pharmaceuticals, 2005). Since the variable region is the only portion with a non-human epitope, there appears to be low antigenicity and increased circulating half-life associated with its administration (Amlot et al., 1995). Following admin-istration, basiliximab rapidly binds to the alpha region of the IL-2 receptor and serves as a competitive antagonist against IL-2. The estimated receptor binding affinity (Ka) is 1 1010 M 1, which is three times more potent than daclizumab (Novartis Pharmaceuticals, 2005). Complete inhibition of the CD25 receptor occurs after the serum concentration of basiliximab exceeds 0.2 mg/mL and inhibition corre-lated with increasing dose (Novartis Pharmaceuticals, 2005; Kovarik et al., 1996). Initial dose findingstudies of basiliximab were similar to daclizumab. Basiliximab, combined with cyclosporine and corticos-teroids, was administered to adult kidney transplant recipients for the prevention of acute cellular rejection. Kovarik et al. (1997) performed a multicenter, open-label pharmacodynamic analysis evaluating basilixi-mab dose escalation in adult patients undergoing primary renal transplantation. Patients received a total of 40 or 60 mg of basiliximab in combination with cyclosporine, corticorticosteroids, and azathioprine. Thirty-two patients were evaluated and were primar-ily young (34 – 12 years), Caucasian (29/32) males (23/32). Basiliximab infusions were well tolerated with not changes in blood pressure, temperature, or hypersensitivity reactions. Thirty patients underwent pharmacokinetic evaluation. Basiliximab blood con-centrations showed biphasic elimination with an average terminal half-life of 6.5 days. Significant intra-and interpatient variability in body weight or observed volume of distribution versus drug clearance was observed. This could not be corrected through body weight adjustment. Gender did not appear to influence the pharmacokinetic parameters of basiliximab, how-ever, this cohort contained a small number of female recipients that may have limited the detection of a difference. Results also indicated that the combination of basiliximab with cyclosporine, corticosteroids, and azathioprine might be inadequate. A total of 22 patients had an acute rejection episode, 16 patients in the 40 mg groups and six in the 60 mg group. These rejections appeared within the first two weeks following trans-plantation, mean time to rejection 11 days. Also three patients experienced graft loss, two of these were immunologically mediated. There was no difference in the basiliximab serum concentration in the patients who experienced rejection versus those who did not. Authors concluded that increased cyclosporine con-centrations, which would inhibit IL-2 production, within the first few days post-transplant may increase the efficacy of basiliximab when used for induction (Kovarik et al., 1996). The clinical efficacy of basili-ximab has been confirmed in several prospective post-marketing trials. Currently, the recommended basiliximab dosing regimen is a total dose of 40 mg, with 20 mg administered 2 hours prior to transplanted organ reperfusion and a subsequent 20 mg dose on post-operative day 4.
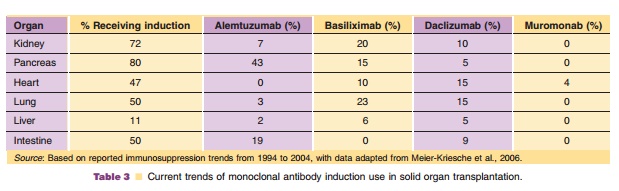
IL-2 receptor antagonists are currently used in all solid organ transplant populations for induction (Table 3), but are only approved for use in kidney transplant recipients. Administration does not reduce the total number of circulating lymphocytes or the number of T-lymphocytes expressing other markers of activations, such as CD26, CD38, CD54, CD69 or HLA-DR (Chapman, 2003). Consequently, it is neces-sary that additional immunosuppressive agents, such a calcineurin inhibitors and antiproliferative agents, be administered as soon as possible to decrease the risk of early acute rejection. The advantage of IL-2 receptor antagonists is that they confer a decreased risk of infusion related reactions, post-transplant infection and malignancy when compared to immu-nodepleting agents. The use of these agents has increased since the introduction of more potent maintenance immunosuppressant agents and they are now the agents of choice in kidney, lung, liver, and pancreas transplant recipients. Although these agents have been evaluated in organ recipients who are at high risk for acute rejection, they are mainly reserved for patients who are at low to moderate risk. Also, these agents are still being evaluated for use in immunosuppression protocols which withdraw or avoid corticosteroids or calcineurin inhibitors. There may be an increased risk of anti-idiotypic IgE anaphylactic reactions in patients who receive repeat courses of IL-2 receptor antagonists. Two published case reports describe patients who had been pre-viously exposed to an IL-2 receptor antagonist and upon subsequent exposure developed dyspnea, chest tightness, rash and angioedema. However, in one case where basiliximab was the offending agent, daclizu-mab was successfully administered following a negative skin test. Therefore caution maybe war-ranted in patients who receive a dose of an IL-2 antagonist without concomitant corticosteroids fol-lowing previous exposure in the past 6 months when circulating antibodies are expected to be present.
Related Topics