Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Monoclonal Antibodies in Solid Organ Transplantation
Alemtuzumab - Specific Agents Used In Solid Organ Transplant
Alemtuzumab
Recently, alemtuzumab was introduced into solid organ transplantation.
Alemtuzumab is a recombinant DNA-derived, humanized, rat IgG1k monoclonal antibody targeting the 21 to 28 kDa cell surface protein
glycoprotein CD52, which is produced in a Chinese hamster ovary suspension
medium (Genzyme Corporation, 2005; Kneuchtle et al., 2004). Initially, the
first anti-CD52 antibodies were developed from rat hybrid antibodies that were
produced to lyse lymphocytes in the presence of complement (Morris, 2006).
Campath-1M was the first agent developed. This molecule was a rat IgM antibody
which produced little biological effect. In contrast, the rat IgG (Campath-1G)
produced profound lymphopenia (Morris, 2006). In order to prevent the formation
of antibodies against the rat IgG the molecule was humanized and called
alemtuzumab or Campath-1H (Morris, 2006). The biologic effects of alemtuzumab
are the same as Campath-1G, and include comple-ment-mediated cell lysis,
antibody-mediated cytotoxi-city, and target cell apoptosis (Magliocca, 2006).
The CD52 receptors accounts for 5% of lymphocytesurface antigens (Morris,
2006). Cells which express the CD52 antigen include T- and B-lymphocytes,
natural killer cells, monocytes, and dendritic cells (Genzyme Corporation,
2005; Bloom et al., 2006). Following administration a marked decrease in circulating
lymphocytes is observed. Use in the hematology population indicates that this
effect is dose-dependent . However, single doses of 30 mg or two doses of 20 mg
are currently used in the solid organ transplant population.
The plasma elimination half life after single doses is reported to be
around 12 days and the molecule may be removed by post-transplant
plasmapheresis (Magliocca, 2006). The
biological activity of alemtuzumab, however, may last up to several months. One
in vivo study of kidney transplant recipients aimed to observe the recovery and
function of lymphocytes following administration of 40 mg of alemtuzumab (Bloom
et al., 2006). Authors reported a 2 Log reduction in peripheral lymphocytes
following administration. Absolute lymphocyte counts at 12 months remained
marketly depleted, falling below 50% of their original baseline. Monocytes and
B-lymphocytes were the first cell lines to recover at 3 to 12 months
post-administration. T-lymphocytes re-turned to 50% of their baseline value by
36 months (Bloom et al., 2006).
Currently, alemtuzumab is only FDA approved for the treatment of B-cell
chronic lymphocytic leukemia. The first report of alemtuzumab use in solid
organ transplantation appeared in 1991. Friend et al. (1991) published a case
series on the use of alemtuzumab to reverse acute rejection in renal transplant
recipients. Shortly thereafter, Calne et al. (1999) issued the first report of
alemtuzumab use as an induction agent. The authors reported the results of 31
consecutive renal transplant recipients. Patients received two 20 mg doses of
alemtuzumab, the first dose was given in the operating room and the second dose
was given on post-operative day 1. Patients were initiated on low dose
cyclosporine monotherapy, with a goal trough range of 75 to 125 ng/mL. Three
patients experienced corticosteroid responsive rejection (20%) and were
maintained on corticosteroids and azathioprine following rejection. Allografts
remained functional in 94% (29/31) of patients at 15 to 28 months post transplant
(Calne et al., 1999).
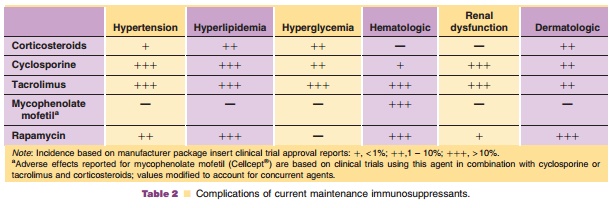
Alemtuzumab is currently being used for induc-tion and for treatment of
rejection (Morris, 2006). In a recent review of immunosuppression trends in the
United States, alemtuzumab use has markedly increase in the past 3 years, with
use primarily limited to induction (Table 2). In 2004, alemtuzumab was the
predominant agent used for induction in both pancreas and intestinal transplant
recipients (Meier-Kriesche et al., 2006). Use in liver transplant has been
limited, buthas appeared in a couple of published trials. Specific findings
from these trials indicate that patients without hepatitis C were able to
tolerate lower levels of calcineurin inhibitors which corresponded to lower
serum creatinine levels at one year post transplant (Tzakis et al., 2004). In
contrast, administration of alemtuzumab positively correlated with early
recur-rence of hepatitis C viral replication (Marcos et al., 2004). Alemtuzumab
induction has allowed for early withdrawal of corticosteroids in several clinical
trials, thereby decreasing long-term exposure which has been correlated with an
increased incidence of cardiovas-cular disease, endocrine and metabolic side
effects. However, early trials in which calcineurin inhibitor avoidance was
initiated, the rate of early acute anti-body-mediated rejection was 17%
compared to 10% under traditional immunosuppression which included calcineurin
inhibitors (Magliocca, 2006).
The infusion of alemtuzumab is well tolerated. In general, induction
doses are administered imme-diately preceeding reperfusion of the transplanted
allograft. Pre-treatment with corticosteroids, diphen-hydramine and
acetaminophen is generally advised to prevent sequelae from cellular apotosis.
However, cytokine release associated with alemtuzumab is insignificant in
comparison to other agents (Morris, 2006).
Currently, there are few published experiences detailing long-term
outcomes in patients who re-ceived alemtuzumab induction (Magliocca, 2006).
Initially clinicians were concerned that the profound lymphodepletion that was
observed following admin-istration would lead to a significant increase in the
number of severe infections. Therefore, lymphocyte response to donor antigens
following alemtuzumab administration was also evaluated in vitro (Bloom et al.,
2006). Lymphocytes from patients treated with alemtuzumab were able to respond
to donor antigens and cytokines. A small subset of patients, however, were
hyporesponsive which is similar to the control patients observed in this study
(Bloom et al., 2006). In addition, several reports detailing the use of
alemtu-zumab thus far suggest that both infection and malignancy rates are
minimal when compared to other agents used for the same indication (Morris,
2006; Magliocca, 2006). At present, the most signifi-cant concern associated
with alemtuzumab adminis-tration is an increased incidence of autoimmune
diseases. The exact incidence and etiology of auto-immune diseases following
alemtuzumab administra-tion in solid organ transplant is currently unknown.
Initial reports of autoimmune diseases associated with alemtuzumab
administration came from the multiple sclerosis population. A single center
observed the development of Grave’s disease in 9 out of 27 patients who
received alemtuzumab (Coles et al., 1999).
Thyroid function in all patients was normal prior to alemtuzumab and the
mean time to development of autoimmune hyperthyroidism was 19 months (range 9
to 31 months) (Coles et al., 1999). Autoimmune hyperthyroidism was first
reported in a kidney transplant recipient who received alemtuzumab in-duction 4
years earlier (Kirk et al., 2006). Watson et al. (2005) recently published a 5
years experience with alemtuzumab induction, in which they reported a 6% (2/33)
incidence of autoimmune disease development following administration. One
patient developed hyperthyroidism in the early post-transplant period and one
patient developed hemolytic anemia which was refractory to corticosteroids.
With the increased use of alemtuzumab in solid organ transplant the actual risk
of autoimmune disease development may be more accurately assessed in the next
decade.
Related Topics