Chapter: Chemistry : Electro Chemistry and Corrosion
Important Questions and Answers: Electro Chemistry and Corrosion
Electrochemistry & corrosion.
01. Define an Electrochemical cell. Give one
example.
A cell
which converts chemical energy into electrical energy is known as
electrochemical cell. Example: Daniel cell , Batteries.
02. What do you mean by redox reaction?
Both
reduction and oxidation takes place simultaneously in a cell reaction then it
is known as redox reaction of an electrochemical cell.
03. What is electrode potential?
It is the
measure of tendency of a metallic electrode to lose or gain electrons, when it
is in contact with its own salt solution. It is denoted as “E”
04. Define an origin of electrode potential.
When a
metallic electrode is placed in its own salt solution, two types of reaction
takes place.
+ve ions may pass into the solution. M → Mn+ + ne-
(oxidation)
+ve ions from the solution may deposit over the
metal. Mn+ + ne-→M (reduction)
The above
reaction takes place in an electrode then it is known as an origin of electrode
potential.
05. Define oxidation potential and reduction
potential.
Oxidation
potential: The tendency of a metallic electrode to lose electrons, Reduction
potential: The tendency of a metallic electrode to gain electrons
06. How an electrochemical is measured? Define EMF
of an electrochemical cell.
It is
measured by EMF.: “The difference of potential which causes flow of electrons
from one electrode of higher potential to the other electrode of lower
electrode potential”.EMF= ER-EL
07. What are the applications of electrochemical
cell?
Determination
of sparingly soluble salt.,Determination of the valency ion. Determination of
standard free energy change and K.
Potentiometric
titrations can be carried out.,Hydrolysis constant can be determined.
08. Define electrochemical series.
When
various types of metallic electrodes are arranged in their increasing order of
standard reduction potential on the basis of hydrogen scale is known as emf
series.
09. Write the significance of electrochemical
series.
To
calculate the standard emf of the cell.,Relative ease of oxidation or
reduction.
Displacement
of one element by the other., Hydrogen displacement behavior. Determination of
equilibrium constant (K) for the reaction.
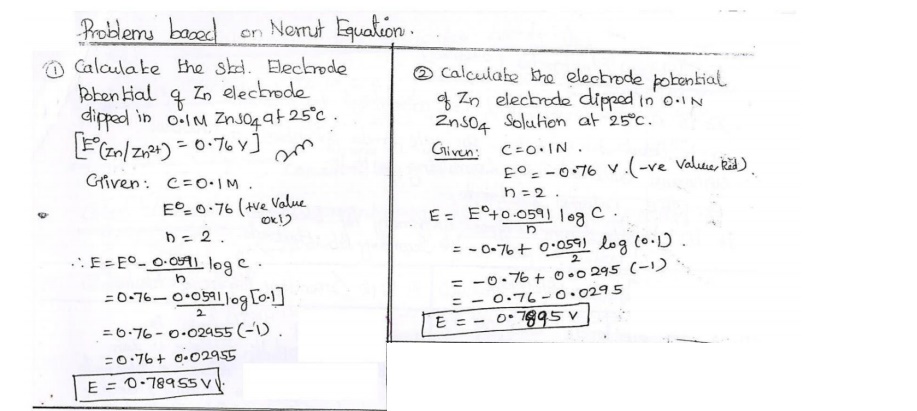
10.Write the mathematical form of Nernst equation
and give one application.
E = Eo +
0.0591/n log [Mn+]
Application:
1.It is used to calculate electrode potential of unknown metal.
Corrosion tendency of metals can be predicted.3.
Applications of emf series.
11.Define corrosion.
The
gradual destruction of the metal or an alloy surface by the chemical or
electrochemical reaction with its environment. (i.e) metal into metal oxide.
12. What are the types of corrosion?
(i).
Chemical corrosion. (ii).
Electrochemical corrosion
13. What are the factors which affect corrosion?
(i).Air and moisture. (ii). Electrolytes in
water.(iii). Presence of impurities in metal. (iv). Presence of gases like CO2
and SO2 (v). Differential aeration.
14. Define chemical corrosion. What are the types
of chemical corrosion?
It is
known as dry corrosion. It is due to the local attack of metal surfaces by the
atmospheric gases like oxygen,H2S,SO2 , N2, etc.
3 types.
(i). Oxidation Corrosion. (ii). Corrosion by other gases. (iii). Liquid-metal
corrosion.
15. Define an Electrochemical
corrosion. What are the types of Electrochemical corrosion?
It occurs due to the existence of separate “anodic”
and “cathodic” areas between which current flows through the conducting
solution. At anodic area oxidation occurs and anodic part of metal is
destroyed. (i). Galvanic corrosion. (ii). Concentration cell / Differential
aeration corrosion.
16. What are the differences
between chemical and electrochemical corrosion?
Chemical corrosion
1) It occurs dry state
2) Local attack to metal by environment
3) Chemical corrosion is self-controlled
4) It follows adsorption mechanism
5) Ex: formation of mild scale on iron surface
Electrochemical corrosion
1) Wet
state (i.e) presence in moisture
2) Large
number of cathodic and anodic areas
3) It is
continuous process
4) It
follows electrochemical reaction
5) Ex:
rusting of iron in moisture
17.What isBedworth rule?
The ratio
of the volume of the oxide formed to the volume of the metal consumed is
pilling bedworth rule
18.Bolt and Nut made of the same
metal is preferred in practice . Why?
Because
such a combination will not permit galvanic corrosion to take place.
19.What are the methods used to
control the corrosion?
1.
Sacrificial anodic method 2.Impressed current cathodic metho
20 What is paint?
Paint is
a mechanical dispersion of one or more finely divided pigments in a medium
(thinner + vehicle) .When paint is applied to a metal surface, the thinner
evaporates, while the vehicle undergoes slow oxidation forming a pigmented
film.
21.Differenciate Electroplating
and Electro less plating
Electroplating
It is
carried out by passing current
Separate
anode is employed
Anodic
reaction is M → Mn+ + ne-
Thickness
of the plating is 1-100μm
Electroless plating
Used
reducing agent
Catalytic
surface of the substrate acts as an anode
Anodic
reaction is R → 0 + ne-
It has a
thickness of 1-100μm
22. Define Electro plating and Electroless
plating
Electro
plating:
It‟s a process in which the coating metal is
deposited on the base metal by passing a direct current through an electrolytic
solution containing the soluble salt of the coating metal.
Electro
less plating:
It is a technique
of depositing of a noble metal from its salt solution on a catalytically active
surface of a base metal by using a suitable reducing agent.
PART B
1. What
is electrochemical cell? Explain with example of Daniel cell.
Electrochemical cell: Cells in which the e-s
transferred due to redox reaction and converted into electrical energy.
Example : Daniel cell.
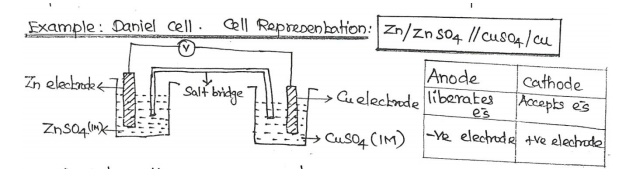
Daniel
cell consists of two electrodes like Zn and Cu.
Zinc
electrode is dipped in 1M ZnSO4 solution and Cu electrode dipped in 1M CuSO4
solution.
The two
solutions are inter connected by a salt bridge and two electrodes are connected
by a wire through the volt meter.
Each
electrode is known as half-cell.
The e-s
liberates from Zn electrode flow through the external wire and is consumed by
copper ions at the cathode.

Salt
Bridge: U- tube containing saturated solution of KCl in agar-agar gel. It
connects two half cells.
Function
of salt bridge: It eliminates liquid junction potential and electrical
continuity between two half cells.
How electrochemical cell is measured by
potentiometrically? Or What is emf? How emf is measured by pogendroff’s method?
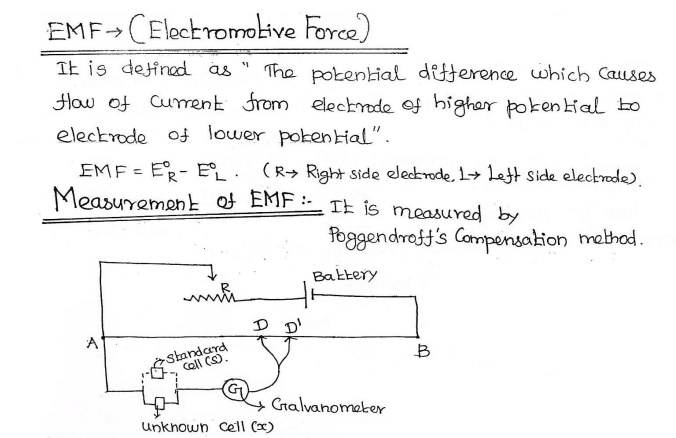

03. What are electrochemical series? Give its
applications.
Electrochemical
series: An arrangement in which the standard electrode reduction potential of
different metals are arranged in increasing order on the basis of
hydrogen
scale is known as emf series.
Applications
of EMF series: (i). To calculate the std. emf of a cell.
(ii).
Relative ease of oxidation or reduction. (iii). Displacement of one element by
other. (iv). To determine K of a reaction.
(v). Hydrogen displacement from acid solutions.
(vi). Predicting spontaneity of
redox reations.
(i). To
calculate the std. emf of a cell.
The emf of a cell is calculated as follows, Ecell = ER-EL
(ii). Relative ease of oxidation or reduction.
+ve value
– higher reduction potential – high reduction. F- = 2.87, higher
reduction – get oxidized
Li+ = -3.07, lower reduction – high oxidation.
(iii). Displacement of one
element by other.
Metals
which lie higher in the series can displace those elements which lie below them
in the series. Cu can easily displaced by Zn
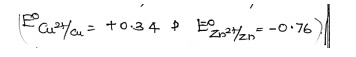
(iv).
Determination of Equilibrium Constant K of a reaction.
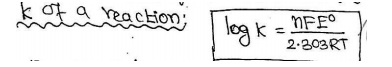
From the
value of Eo, we can be determined the equilibrium constant K (v)
Hydrogen displacement Behavior
Metals
with above H2 scale will displace the hydrogen from an acid solution (ie)-ve
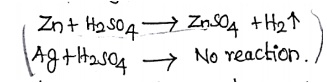
reduction potential. Zinc reacts with hydrogen but not in Ag. Why?
Because
it has positive reduction potential
(vi)
Predicting Spontaneity of Redox
Reactions:
Eo
value is positive=Reaction is Spontaneous
Eo
value is –ve =Non-Spontaneous Process.
![]() 04. Derive Nernst equation.
04. Derive Nernst equation.
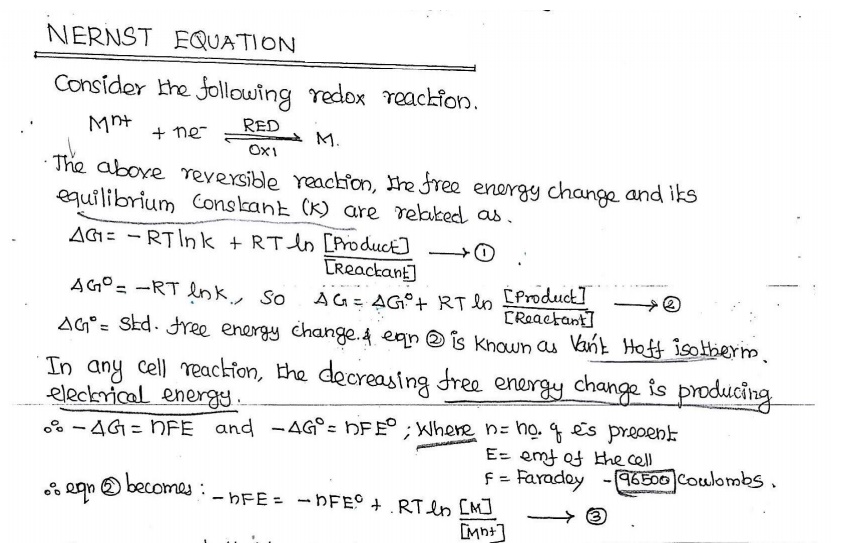
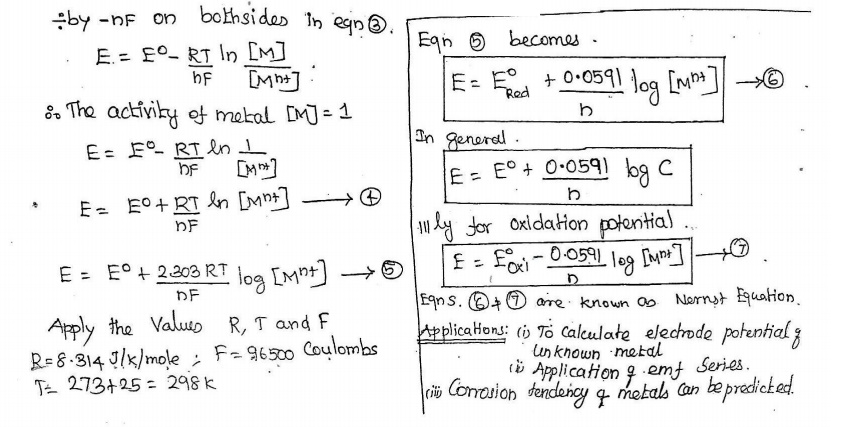
Applications:
To calculate electrode potential of unknown metal
Application of emf series
Corrosion tendency of methods can be predicted
What is Chemical corrosion? Explain with its types.
It is
classified into 3 types. i). Oxidation corrosion.
ii).Corrosion
by the other gases – Hydrogen iii).Liquid – Metal corrosion
01. Oxidation
corrosion
Oxygen
present in atmosphere attacks metal surface resulting in the formation of
metallic oxide which is a corrosion product and known as oxidation corrosion.

Oxidation
occurs first at the surface of the metal and the resulting metal oxide scale
forms a barrier that tends to restrict either further oxidation. For oxidation
to continue either the metal must diffuse outwards through the scale to the
surface or the oxygen must diffuse inwards through the scale, to the underlying
metal.
Both
transfer occurs, but the outward metal diffusion is generally, much more rapid
than the inward diffusion of oxygen. Since the metal ion is appreciably smaller
than the oxygen ion and consequently of much higher mobility.
02.
PILLING – BEDWORTH RULE:
The ratio
of the volume of the oxide formed to the volume of the metal consumed is
pilling bed worth rule.
03.
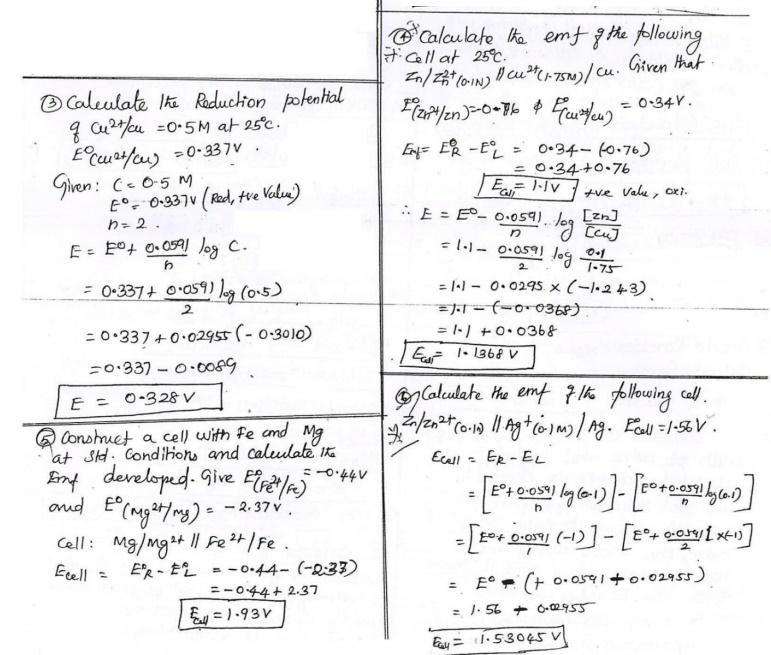
Corrosion by other gases – Hydrogen.
i) At
ordinary Temperature : Hydrogen Embrittlement.
The
process of formation of cracks and blisters on the metal surface, due to high
pressure of hydrogen gas is called hydrogen embrittlement
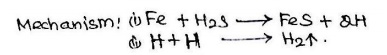
Metal direct contact with H2s and forms atomic hydrogen
l direct contact with H2s and forms atomic hydrogen
Atomic hydrogen diffuses readily into metal to form
molecular hydrogen
At high temperature – Decarburization
The
process of decrease in carbon content in steel is termed as decarburization of
steel.

04)
Liquid – metal corrosion
This is
due to the chemical action of following liquid metal at high temperature. The
corrosion reaction involves
Either
dissolution of a solid metal by a liquid metal.
Liquid metal may penetrate into the solid metal.
Explain
Electrochemical corrosion with its types.
01.
Galvanic corrosion:
The
corrosion occurs when two different metals are in contact with each other in
presence of an aqueous electrolyte solution or moisture is known as Galvanic
corrosion.
The more
active metal acts an anode the less active and metal acts as a cathode
In Zn-Fe
bimetallic couple, Zn undergoes to corrosion, because compare with Fe, it has
higher –Ve emf Value. So it acts as an anode. Iron acts as cathode and it is
protected.
But in
the case of Fe – copper bimetallic couple Fe dissolves and copper is protected.
(ie) Fe acts an anode and copper as a cathode.
Prevention
of Galvanic corrosion:
To prevent
Galvanic corrosion, the bolt and but are made by same metal. If they are n‟t
corrosion will take place easily.
It may be
minimized by putting an insulating material between the two metals.
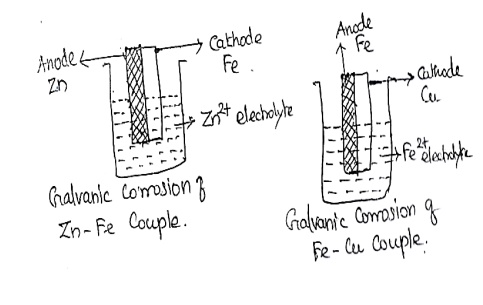
02.
Differential aeration corrosion:-
Otherwise it is known as concentration cell corrosion.
It is due
to the formation of concentration cell formed by the variation of concentration
mainly of oxygen or any electrolyte on the surface of the base metal.
i).When a
metal is partially immersed in a solution, the metal inside the solution has
very poor aeration compared with the metal that is outside the solution.
ii).The difference in the air concentration of
the base metal can produce the anode with less aerated area and cathode.
iii).Corrosion
will take place at the anode area where the metal is converted into metal ion.
Example: A zinc metal partially dipped in a brackish solution.
Example:
Pitting Corrosion - The holes are formed at corrosion in concentrated places.
Low oxygen part= anode , More oxygen part=cathode.
It is a
localized attack, resulting in the formation of a hole around which the metal
is relatively unattached. Ex: metal area covered by a drop of water, sand,
dust, etc.
07. Distinguish between Chemical corrosion and
Electrochemical corrosion.
Chemical corrosion
1) It occurs dry state
2) Local attack to metal by environment
3) Homogeneous metal surface gets corroded
4) Corrosion products accumulate in the same
place, where corrosion occurs
5) Chemical corrosion is self-controlled
6) It follows adsorption mechanism
7) Ex: formation of mild scale on iron
surface
8) 3 types. i). Oxidation corrosion
ii). Corrosion by other gases- H2
iii). Liquid – Metal corrosion.
Electrochemical corrosion
1) Wet
state (i.e) presence in moisture
2) Large
number of cathodic and anodic areas
3)
Heterogeneous surface (i.e) Bimetallic contact.
4)
Corrosion occurs at the anode, while the products formed else where
5) It is
continuous process
6) It
follows electrochemical reaction
7) Ex:
rusting of iron in moisture
8) 2
types.
i).
Galvanic corrosion.
ii).
Differential aeration corrosion.
08. What are the factors influencing corrosion?
The rate
of corrosion is mainly depends on i)
Nature of
the metal
Nature of the environment
i). Nature
of the metal
a).
Position in emf series: Metals above hydrogen in emf series, corroded
easily.
Metals
have high –ve reduction potential undergoes corrosion.
Relative areas of the anode and cathode: Rate of
the corrosion has higher % in anodic area, the rate of corrosion will be more,
when the cathodic area is larger.
Purity of the metal: The 100%
pure will not undergo any type of corrosion. If
impurity
present in higher percentage, corrosion takes places at anode.
d) Over
voltage: Corrosive environment is inversely proportional to corrosion rate. e)
Nature of the surface film: It is known as pilling- bed worth rule.
f) Nature
of the corrosion product: Corrosion is faster when the corrosion product is
soluble in corroding medium. If the corrosion product is volatile, the
corrosion rate will be faster.
ii).
Nature of the environment:
i).
Temperature: Rate of corrosion α temperature.
The rate
of corrosion increase with temperature
ii).
Humidity: If the humidity is high in the environment, corrosion will be more.
iii).
Presence of corrosive gases: The acidic gases like
CO2,SO2,H2S are produce electrolytes, which are acidic and increases the
electrochemical corrosion.
iv).
Presence of suspended particles: Particles like NaCl present in
the moisture and acts a
powerful
electrolyte and then enhance the electrochemical corrosion.
v).
Effect of pH; the rate of corrosion will be maximum when the
corrosive environment is acidic (ie) PH<7.
Explain the Sacrificial anode and impressed current
techniques for the preventions of corrosion.
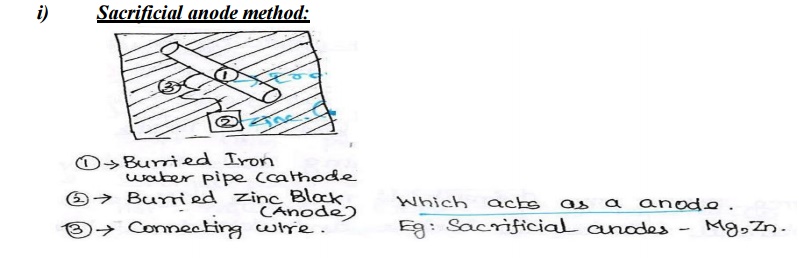
i). The
application of sacrificial anodes in cathodic protection is based on the
differences in electrochemical reactivity of metals.
ii). In
this method, the metal to be protected from corrosion is connected to more
active metal which acts as an anode.
iii). In
a redox reaction involving iron and zinc, the zinc will serve as the anode, and
iron the cathode.
iv). The
zinc anode will oxidize and provide electrons for the reduction of Fe2+
to elemental iron. v). This is called cathodic protection. The zinc anode is
termed a sacrificial anode.
vi). Iron
pipes buried in the ground, and designed to carry water, would normally be
expected to rust pretty quickly.
vii).If
they are buried along with a piece of zinc, and connected by a wire the zinc
will provide cathodic protection.
2.
Corrosion control through Impressed current method:
An
alternative method of providing the current to protect a system is to use some
sort of external power supply. As with the sacrificial system , the structure
to be protected is made the cathode, the difference being that the driving
force. Behind the current is not the difference in potential between the anode
and cathode of the system but from the power supply.
Both
these types of cathodic protection may be applied to buried pipelines and to
steel hulled ships. For oil drilling platforms, however the method employed is
sacrificial in the vast majority of cases in the north sea.
This
technique is useful for protecting large structures like water tanks,
underground oil collers, laid up ships,etc.
10. What is paint? What are the constituents and
their functions in paint?
Paint: paint is a mechanical dispersion
of one or more finely divided pigments in a medium (thinner + vehicle) . When paint is applied to a
metal surface, the thinner evaporates, while the vehicle undergoes slow
oxidation forming a pigmented film.
Constituents:
1.
Pigments. 2. Vehicle or drying oil. 3. Thinner. 4. Drier. 5. Filler or
extender. 6. Plasticizer 7. Antiskinnig agent.
i) Pigments: They are colour producing
substances in paint.
Ex: white
colour- white lead, black=- carben black red-indian red, green – chromium
oxide.
Function: i). It gives colour and opacity to the
film. ii). It also provides strength to the film.
iii). It
provides weather resistance of the film.
ii) Vehicle
(or) drying oil: This is a non-volatile portion of a medium and film
forming constituent of the paint. These are high molecular weight fatty acids
present in vegetable and animal oils. Ex: Linseed oil, castor oil.
Function:
They form a protective film by the oxidation and
polymerization of the oil ii) They hold the pigment particles together on the
metal surface
They impart water repellency , toughness and
durability to the film.
Thinners or solvents: Thinner
is a volatile substance present in the medium.
Ex: turpentine, toluol, xylol etc.
Function:
It increases the elasticity of the film
II) It helps easy drying of the paint
film
It increases the penetrating power of the vehicle.
Extenders or fillers: These
are colourless (white) pigments which improve the quality of the paint. Ex:
Gypsum, Jal ,china clay, silica, etc.
Functions:
It reduces the cost of paint
It prevents the cracking and shrinkage of the film
iii) It modifies the shades of the pigments
v).Driers: these are the substances, used
to accelerate the process of crying. Ex: metallic soaps.
Functions: i).They are oxygen –carriers (or) catalists.
ii). They
provide oxygen , which is essential for oxidation, polymerization of drying
oil. vi).Plasticisers: these are chemicals added to the paint to provide
elasticity to the film and to prevent cracking of the film. Ex: triphenyl
phosphate, tricresyl phosphate, etc.
vii).
Antiskinning agents: These are chemicals added to the paint to prevent
gelling and skinning of the paint. Ex: polyhydhroxy phenol.
11. Explain the electroplating of Gold.
Electroplating: It‟s a
process in which the coating metal is deposited on the base metal by passing a
direct current through an electrolytic solution containing the soluble salt of
the coating metal.
Ex:
Electroplating of Au (gold) over copper object.
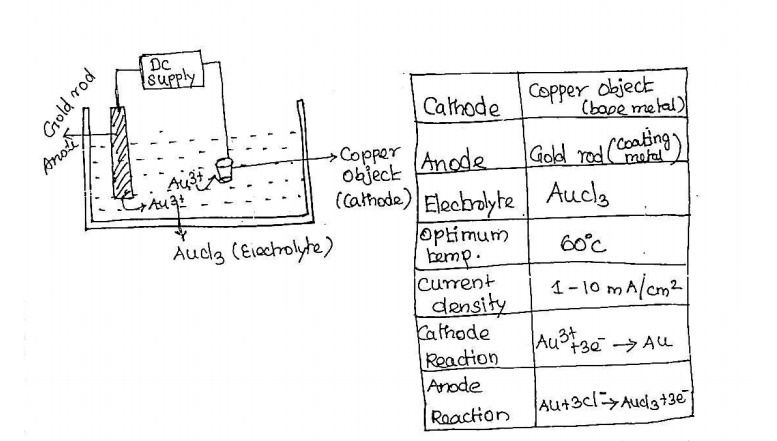
The
copper object to be plated is first treated with dilute acid.
Then this
copper object and gold rod are immersed in an electrolyte. Copper object acts
cathode and gold rod is an anode.
These
electrodes are connected with direct power supply.
When
current is passed from battery through the solution, gold dissolves in
electrolyte and deposits uniformly in copper object.
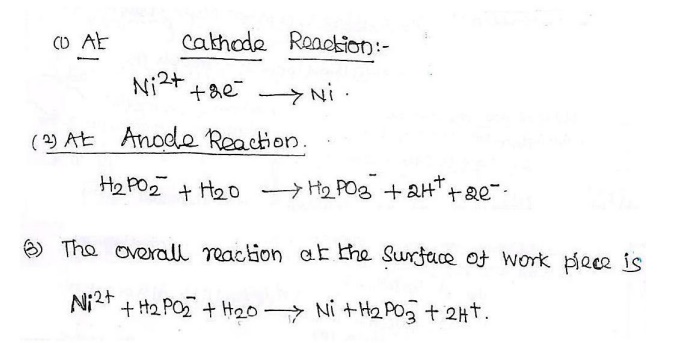
12. Explain the Electroless plating of Nickel.
Metal
ions + Reducing Agent Metal (Deposited) +
Oxidised Product
Definition: it is a
technique of depositing of a noble metal from its salt solution on a
catalytically active surface of a base metal by using a suitable reducing
agent.
Electroless
Nickel plating:
Step 1:
Pretreatment and activation of the surface:
The
surface to be plated is first degreased by using organic solvents or alkali
followed by acid treatment.
Step 2:
preparation of plating bath composition:
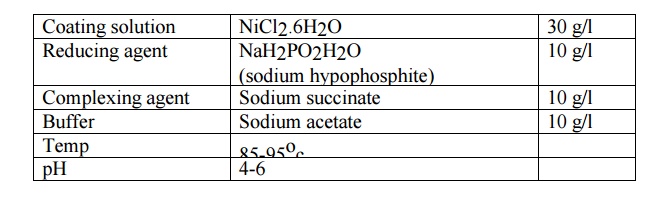
The
pretreated object is immersed in the plating bath for the required time during
which the following reduction reaction will occur and the Ni gets coated over
the object.
GLOSSARY
CELL:
Cell is an assembly of two electrodes and an electrolyte. It
consists of two half cells. Each half cell contains an electrode material in
touch with electrolyte.
CURRENT:
Current is flow of electricity through a conductor. It is measured in ampere.
ELECTRODE:
Electrode is a material or a metallic rod/bar/strip which conducts electrons.
ANODE:
Anode is an electrode at which oxidation occurs.
CATHODE:
Cathode is an electrode at which reduction occurs.
ELECTROLYTIC
CELLS: Electrolytic cells are cells in which electrical energy is converted
into chemical energy.
ELECTOCHEMICAL
CELLS: Electrochemical cells are cells in which chemical energy is converted
into electrical energy.
SALT
BRIDGE: Salt bridge consists of a U – tube containing a gel saturated with KCL
or NH4 NO3 in agar – agar. It consists the two half cells of the
galvanic cells by the electron movement through it.
OXIDATION
POTENTIAL: Electrode lose electrons REDUCTION POTENTIAL: Electrode gain
electrons.
SINGLE
ELECTRODE POTENTIAL: Measure of the tendency of a metallic electrode to lose or
gain electrons, when it is in contact with a solution of its own salt.
STANDARD
ELECTODE POTENTIAL: Measure of the tendency of a metallic electrode to lose or
gain electrons, when it is in contact with a solution of its own salt of a 1
molar concentration at 250C.
ION –
SELECTIVE ELECTRODES: These are electrodes which have the ability to respond
only to a particular ion and develop potential, ignoring the other ions in a
mixture. The potential developed by an ion – selective electrodes depends only
on the concentration of particular ions.
ELECTROCHEMICAL
SERIES: An increasing order of the standard reduction potentials CORROSION:
Corrosion is defined as the gradual destruction of metals by the chemical or
electrochemical reaction with its environment.
PILLING
BEDWORTH RATIO; The ratio of the volume of the oxide formed to the volume of
the metal consumed is called ”Pilling – Bed worth ratio” or “Pilling – Bed
worth rule”.
PAINT:
Paint is a uniform dispersion of finely dived pigments, filters and driers in a
liquid called medium (thinner + vehicle ).When a paint applied to a metal
surface , the thinner evaporates, while the vehicle undergoes slow oxidation
forming a pigmented film.
Related Topics