Chapter: Chemistry : Electro Chemistry and Corrosion
Electro Chemistry
ELECTRO CHEMISTRY
1 Introduction
1.1 Types of cells
2 Electrochemical Cells
2.1 Reversible and Irreversible cells
3 Electrode Potential
3.1 Reduction Potential
3.2 Single electrode Potential
3.3 Standard electrode Potential
3.4 Measurement of Standard electrode Potential
4 EMF of Cell
4.1 Measurement of EMF of cell
4.2 Applications of EMF measurement
5 Electrochemical Series
5.1 Significance of EMF series
6 Nernst Equation
6.1 Nernst equation for reversible cell
6.2 Problems based on Nernst equation
1 INTRODUCTION
Electrochemistry is the branch of science which
deals with the relationship between chemical reaction and electricity.
Electrochemistry is the study of the process involved in theinterconversion of
electrical energy to chemical energy and vice – versa
Electric current is a flow of electrons generated
by a cell or a battery when the circuit is completed. A substance which allows
electric current to pass through it ,is called a conductor, e.g. metals,
graphite, fused metallic salts while non – conductor is a substance which does
not conduct the electric current e.g. plastics, wood, non – metals ect,
IMPORTANT
TERMS INVOLVED IN ELECTROCHEMISTRY
Cell:
Cell is an assembly of two electrodes and an electrolyte.Generally, it consists
of two half cells. Each half cell contain an electrode material in touch with
electrolyte.
Current:Current
is a flow of electricity through a conductor. It is measured in ampere.
Electrode is a material rod/bar/strip which conduct electrons.
Anode:
Anode is an electrode at which oxidation occurs
Cathode:
Cathode is an electrode at which reduction occur
Electrolyte:
Electrolyte is a liquid or solution that conducts electric current. There are
three types of electrolytes.
(a) Strong electrolytes: these are the substances
which ionize completely at any concentration.
Example:
HCL,aqueous solutions of NAOH, NaCl and KCL
(b)Weak
electrolytes: Weak electrolytes are the substance which ionize partially in
solution.
Example: CH
COOH, NH OH and aqueous solution of Na CO
Non electrolyte: Non electrolyte are the substances
which do not ionize at any dilutions.
Example: Glucose, Sugar,alcohol, petrol,etc.
Galvanic (or) Voltaic (or)
Electrochemical cell
It is a device that produces electrical energy at
the expense of chemical energy produced in a reaction.
A cell
consists of two half cells or electrodes.
A half-cell or electrode contains a metal rod
dipped in an electrolytic solution. Electrolytic cell:
It is a
cell in which
electrical energy brings
about a
chemical reaction.
Electrochemical cell: It is a
device that produces electrical energy at the expense of chemical energy produced in a reaction.
Differences between electrolytic cell and
electrochemical cell.
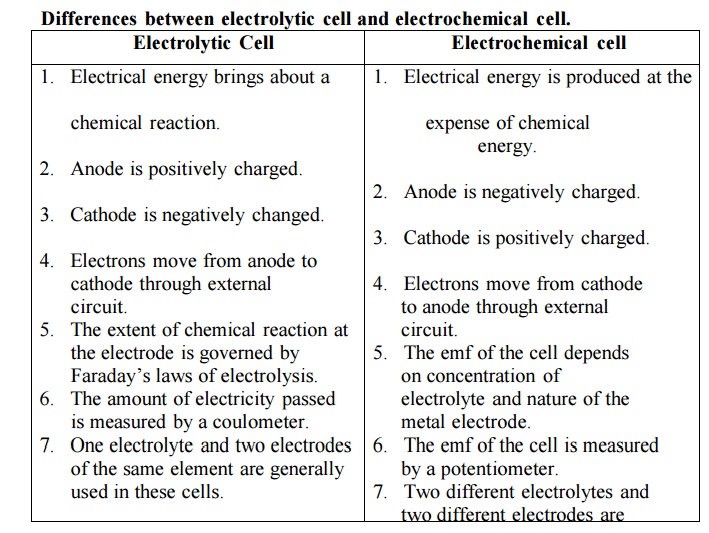
Electrolytic Cell
1. Electrical energy brings about a chemical
reaction.
2. Anode is positively charged.
3. Cathode is negatively changed.
4. Electrons move from anode to cathode
through external circuit.
5. The extent of chemical reaction at the
electrode is governed by Faraday‟s laws of electrolysis.
6. The amount of electricity passed is
measured by a coulometer.
7. One electrolyte and two electrodes of the
same element are generally used in these cells.
Electrochemical cell
1. Electrical energy is produced at the
expense of chemical energy.
2. Anode is negatively charged.
3. Cathode is positively charged.
4. Electrons move from cathode to anode
through external circuit.
5. The emf of the cell depends on
concentration of electrolyte and nature of the metal electrode.
6. The emf of the cell is measured by a
potentiometer.
7. Two different electrolytes and two
different electrodes are
1.1 TYPES OF CELL
Electrolytic cell
It is a
device in which chemical reaction proceed at the expanse of electrical energy.
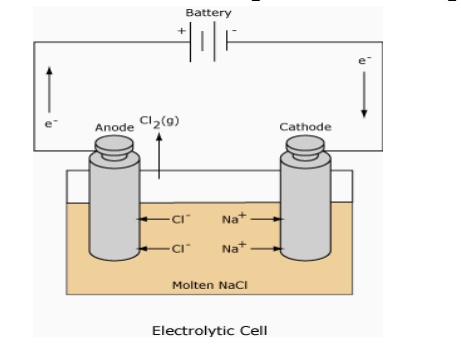
Ex. Electro plating and electrolysis Electrolysis of NaCl
The cell is constituted by dipping two platinum electrode in an
appropriate electrolyte ( NaCl in water ) . The electrodes are connected to the
two terminals of a battery. The electrode connected to positive terminal acts
as anode (attracts anions) and the other electrode connected to the negative
terminal acts as cathode (attracts cations). Chlorine is liberated at anode and
hydrogen is liberated at cathode
Cell reaction:
At anode: 2 Cl
--- > Cl2 ↑ + 2e
At cathode: (i)
Na + + H2O ---
> NaOH + H+
(ii) 2 H+ + 2e -- -- > H2↑
Net reaction:
2NaCl + 2H2O ▬▬► 2NaOH
+ H2 + Cl2
ECTROCHEMICAL CELL:
●consists of two half-cells joined by a salt
bridge or some other path (porous membrane)that
allows ions to pass between the two sides in order to maintain electro
neutrality.
oxidation occurs at one half
cell while reduction takes place at the
other half cell.
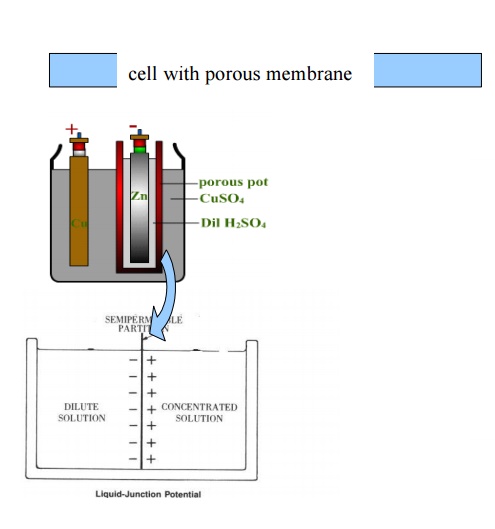
Note:Liquid junction potential is higher when
porous membrane is used for joining two half cells while it is minimum
in a cell with a salt bridge.
Liquid junction potential occurs
when two solutions of different concentrations/compositions are in
contact with each other through porous membrane. It is due to formation of an
electrical double layer of positive and negative charges at the junction of the
two solutions as the ions diffuse through membranes.
Salt bridge: Contains a solution of a salt
(KCl or KNO 3 or NH4NO3) that literally serves as a bridge to
complete the electric circuit, maintain electro neutrality of electrolyte
and
minimize the liquid junction potentia. For precise measurements of potential, a
salt bridge is used.
Fundamental components of electrochemical cell
1.Anode: oxidation half-reaction takes place ;
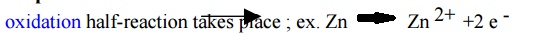
Oxidation
of metal releases metal ions into the solution of oxidation half- cell, leaving behind the electron at the
surface of the metal electrode.
2.Cathode: reduction half-reaction occurs;
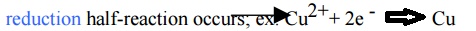
Movement
of metal ions from the solution to the electrode to gain es in reduction half
cell leads to accumulation of positive charge at the surface of the electrode.
Electrolyte:Internal conducting environment
that allows ions to migrate between both half cells so as to
preserve electro neutrality
External circuit : Two
half-cells are joined together by wire through which electrons flow.
Salt bridge/porous membrane: Serves as
a bridge to complete the
electric
circuit and maintain electro neutrality in the electrolyte.
2 Electrochemical Cell or
Galvanic cell
It is a
device in which a redox reaction is used to derive electrical energy. During
the working of the cell the stored chemical energy decreases and this decrease
is gained as electrical energy.
In the
electrochemical cell the electrode at which oxidation occurs is called anode (−
ve) and the electrode at which reduction occurs is called cathode (+ ve).
Example: Zn acts as anode and Cu acts as cathode in
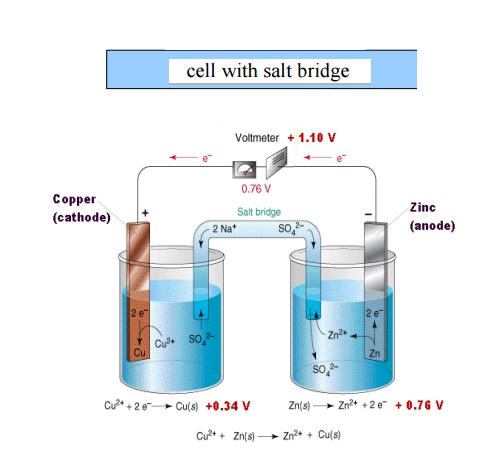
Daniel cell
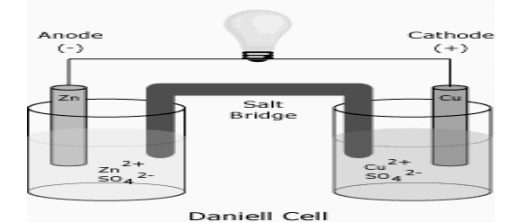
It consists of zinc electrode dipped in 1M zinc sulphate solution and a copper electrode dipped in 1M copper sulphate solution. Each electrode acts as a half cell connected by a salt bridge through a voltmeter. The two solutions can seep through the salt bridge without mixing.
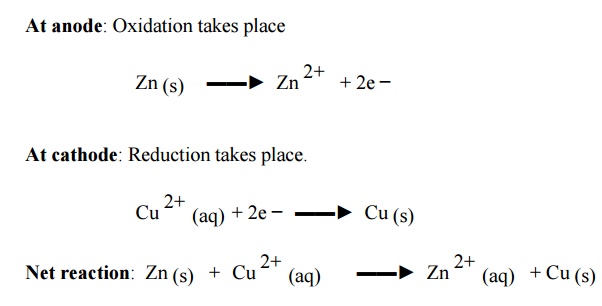
Representation of a galvanic cell
Galvanic cell consists of two electrodes, anode and
cathode
The anode is written on the left hand side while
the cathode is written on right side.
The anode is written with the metal first and then
the electrolyte .The two are separated by a vertical line or semicolon
Ex: Zn / Zn 2+
(or) Zn / ZnSO4 (or) Zn; Zn 2+
(iv) The
cathode is written with electrolyte first and then the metal both are separated
by vertical line or semicolon
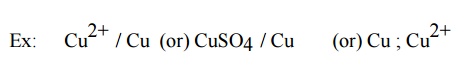
(v) The
two half cells are connected by a salt bridge which is indicated by two
parallel lines.
Salt bridge: It
consists of a U tube filled with a saturated solution of KCl or (NH4)2NO3
in agar-agar gel. It connects the two half cells and performs the following
functions
It eliminates the liquid junction potential.
It provides path for the flow of electrons between
two half cells.
Completes the circuit.
Maintains electrical neutrality in the two
compartments by migration of ions through the porous material thus ensures the
chemical reactions proceed without hindrance
Prevents mixing of the electrode solutions
2.1 Reversible cells
A cell
works reversibly in the thermodynamic conditions. Ex. Daniel cell, Secondary
batteries, Rechargeable batteries.
The cell
is reversible if it satisfies all the following conditions:
If applied emf is equal to derived emf then the net
reaction is zero
If applied emf is infinitesimally smaller than the
derived emf then the cell should act as electrochemical cell (forward reaction)
If applied emf is infinitesimally greater than the
derived emf then the cell should act as electrolytic cell (reverse reaction)
Irreversible cells
Cells
which do not obey the (above) conditions of thermodynamic reversibility are
called irreversible cells. If one of the products escapes from the cell then
that cell cannot be made reversible by applying an external current.
Ex.
Zinc-Silver cell, Primary cells
Zn –Ag
Cell
Zn / H2SO4
(aq) / Ag
Cell reaction:
When two
electrodes are connected from outside, zinc dissolves liberating hydrogen gas.
Since one of the product hydrogen escapes, the cell reaction cannot be reversed
when connected to an external EMF. The cell does not obey the conditions of
reversibility and is called irreversible cell.
3 Electrode Potential :
3.1 Oxidation potential
When a metal M is dipped in its salt solution, one
of the following reactions occurs depending on the metal :
Positive
metal ions pass into the

(e.g.)
When Zn rod is dipped in ZnSO4 solution, Zn goes into solution as Zn2+.
The
electrons attach to Zn rod, giving it a negative charge. The negative charge on
the rod attracts positive ions from solution. Thus a double layer of ions is
formed close to the rod.
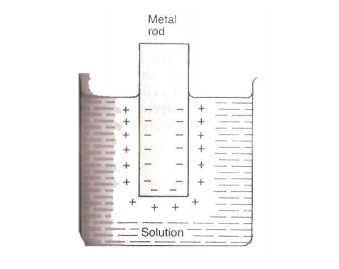
3.2
REDUCTION POTENTIAL
2. Positive ions from the solution deposit over the metal.

When Cu
rod is dipped in CuSO solution, Cu+2 ions from the solution deposit
on metal rod. They attract negative ions from solution. Thus a double layer of
ions is formed close to the metal rod. This is called Helmholtz double layer.
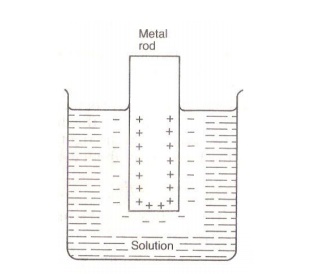
As a result a potential difference is set up
between the metal and the solution. The equilibrium value of the potential
difference is known as electrode potential.
Factors affecting electrode potential or emf of
Cell :
nature of the metal
temperature
concentration of metal ions in the solution
3.3 Single electrode potential (or) electrode
potential :
It is a measure of the tendency of the metal electrode
to lose or gain electrons, when it is in contact with its own salt solution. It
is developed due to the formation of a double layer around the metal rod.
3.4 Standard electrode potential :
It is a measure of the tendency of the metal
electrode to lose or gain electrons, when it is in contact with its own salt
solution of 1M strength at 25C.
Measurement of single electrode
potential :
It is impossible to determine the value of a single
electrode potential. But we can always measure the potential difference between
two electrodes using a potentiometer, by combining the two electrodes to form a
cell. For this purpose we reference electrode. Standard hydrogen electrode is
called primary reference electrode. Calomel electrode is called secondary
reference electrode.
Standard hydrogen electrode (SHE)
(Primary Reference Electrode)
It has a
platinum foil connected to platinum wire and sealed in a glass tube. The
platinum foil is dipped in 1M HCl. Hydrogen gas 1 atm pressure is passed through
the side arm of glass tube as shown in the figure. The standard electrode
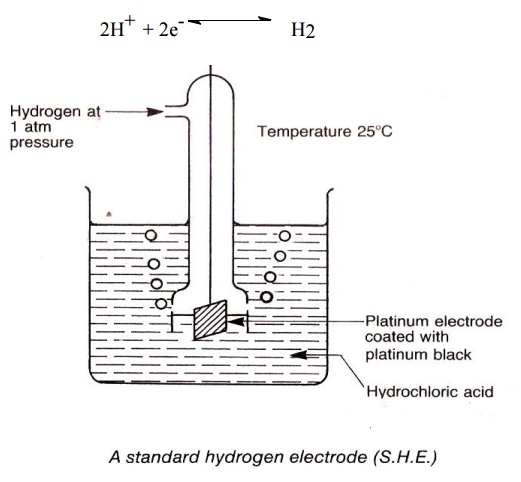
potential of SHE is taken as zero. The electrode is represented,
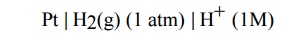
The
electrode reaction is
Limitations (or) drawbacks of
SHE:
H2 gas reduces many ions like Ag+ and
affects compounds of Hg, Ag etc.
It is difficult to get pure H2.
The pressure of H2 is to be kept 1 atm all the
time.
It is difficult to set up and transport.
The electrode potential changes with barometric
pressure.
6) A large
volume of test solution is required.
It cannot be used in solutions of redox systems.
The solution may poison platinum surface. So we use
a secondary reference electrode.
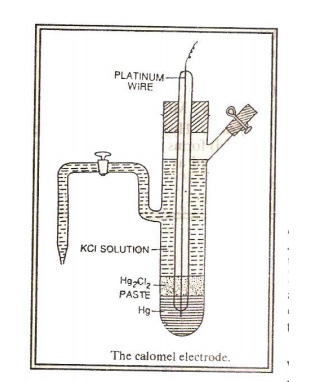
Calomel Electrode (Secondary reference electrode):
It consists of a glass tube containing pure mercury
at the bottom. A paste of mercurous chloride covers the mercury. A solution of
potassium chloride is present over the paste. The bottom of the tube is sealed
with a platinum wire. There is a side tube for electrical contact. The electrode
is represented as,
Hg |
Hg2Cl2(s) | KCl(aq)
The
electrode reaction is,
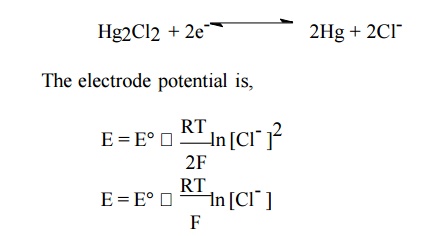
At 25oC,
E = E – 0.0591
log (Cl-)
For saturated KCl, E = +0.242 volt.
Measurement of single electrode
potential using a reference electrode (saturated calomel electrode):
The given electrode, say zinc electrode, is coupled
with saturated calomel electrode as in the figure. Since the reduction
potential of zinc electrode less than that of calomel electrode, zinc acts as
anode and calomel as cathode. The cell reaction will be
Zn/ ZnSO4
(1 M) // KCl (satd )/ Hg2Cl2/Hg
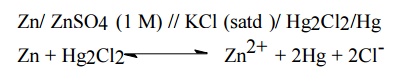
The emf
of the cell is measured using a potentiometer.
The value of Ecell = 1.002 volt.
Now, Ecell = EOright
– E0left
= EOcal
– EOZn
1.002 = 0.242
– E0Zn
EZn =
0.242-1.002
EZn = - 0.76
volt.
Advantages of Reference Electrode
(Calomel Electrode):
Easy to set up.
Easily transportable
Long shelf life
Reproducibility of emf
Low temperature coefficient
Electrode can be used in a variety of solutions.
Eo value is accurately known.
Ion sensitive electrode :
Ion sensitive electrodes have the ability to
respond only to a specific ion and develop a potential ignoring other ions in
the solution.
The types (classification) of
ion-sensitive electrode :
Glass membrane electrodes
Solid state electrode
Pungor or precipitate electrodes
Liquid – liquid electrode
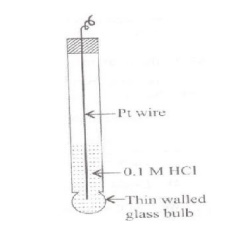
Glass Electrode (or) Measurement
of pH using glass electrode :
Glass electrode contains a thin-walled glass bulb.
The glass has low melting point and high electrical conductivity. 0.1M HCl is
present in the bulb. A platinum wire is inserted in the acid.
When the glass membrane separates two solutions differing in pH, exchange of H+ ions takes place between the solutions. As a result a potential is developed across the membrane. The potential EG is given by,
EG = EG + 0.0591 pH
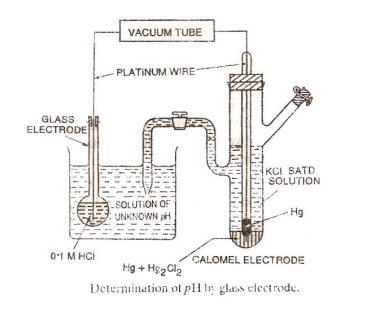
Measurement of pH :
The glass electrode is dipped in the given
solution. This system is connected to saturated calomel electrode as in the
figure. The emf of the resulting cell is measured using a potentiometer.
From the
emf, the pH of the solution is calculated as
below: Ecell =
Eright – Eleft
Ecell=
Ecal – Eglass
Ecell =
0.242 – (EG° + 0.0591v pH)
Ecell =
0.242 - EG° - 0.0591 pH
PH
=Ecell – 0.2422+E0Glass/0.0592
Advantages
of Glass Electrode :
i)It is easily constructed and used
ii)Results
are accurate iii)Electrode is not easilpoisoned iv) Equilibrium is quickly
attained
v) It can
be used in strong oxidizing
solutions, coloured solutions
and
in
presence of metal ions
vi) Using
special glass electrode, pH can be measured from 0 to 12.
vii) It
is used in chemical, industrial, biological and agricultural laboratories.
Disadvantages or Limitations :
i) Glass has high resistance. So special electronic potentiometer
must be used.
It cannot be used in highly alkaline solutions, in
pure ethanol or in acetic acid. If the solution pH is more than 12, glass
membrane is affected by cations.
Applications of Ion-sensitive
electrode :
To determine ions like H+, K+,
Li+, etc.
To determine hardness of water (Ca+2 and
Mg+2 ions)
To determine concentration of F-, NO3-,
CN- etc.
To
determine concentration of a gas using gas-sensing electrodes.
v) To
determine pH of a solution using H+ ion sensitive electrode.
4 EMF of
an electrochemical cell
Difference of potential which causes the flow of
current from the electrode of higher potential to one of lower potential
It is the algebraic sum of the single electrode
potentials provided the proper sign are given according to actual reaction
taking place on the
Nernst
eqn for electrochemical cell
ECell=E right-Eleft, or Ecell
=E cathode -Eanode
E° cell+ 0.0592Vlog[reactant]/[p roduc)
4.1Instrument:
emf of
the cell can be measured by potentiometer-using Poggendorff’s compensation
principle(wheatstone's bridge).
Voltmeter
cannot be used to determine accurate value of emf because during measurement a
part of cell current is drawn which causes change in the emf.
Factors affecting the emf of a
cell
1.Nature of the electrolytes and
electrodes. 2.Concentration and
composition of the electrolytes. 3.pH and temperature of the solution.
4.2 Applications of EMF
Measurements
The
valency of an ion can be determined.
Solubility
of a sparingly soluble salt can be determined.
Potentiometric
titrations can be carried out.
Hydrolysis
constant can be determined.
Determination of standard free energy change and
equilibrium constant
Determination of pH by using a standard hydrogen electrode.
5 ELECTROCHEMICAL
SERIES (e.m.f series)
A series
in which elements are arranged in the ascending (increasing ) order Of their
standard reduction potential is called emf series.
5.1 Significance of EMF series
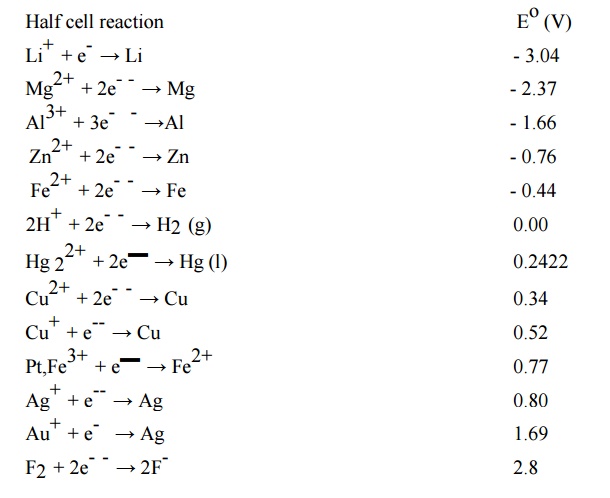
Application / Significance of
electrochemical series
(i) Relative ease of oxidation or
reduction
• The metals which lie above hydrogen in the series
undergo spontaneous oxidation and the metals which lie below SHE undergo
reduction spontaneously ( ie. Acts as Anodes and Cathodes respectively)
• The metals which lie above hydrogen are good
reducing agents and which lies below hydrogen will act as good oxidizing agents
(ii) Replacement tendency
The metal lying above in emf series displaces the
metal lying below it from an electrolyte of the later.
Example
1: Ni spatula cannot be used to stir copper sulphate solution due to the
following reaction
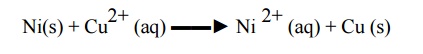
Example 2: when zinc is dipped in copper sulphate solution copper gets deposited (displaced)

(iii) Liberation of Hydrogen
•
The metal with negative reduction potential will displace H2 from an
acid solution
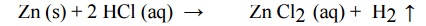
Hence
acids cannot be stored in galvanized steel containers.
For
exactly the same reason galvanized steels are not used to store food stuffs
containing vinegar. (Vinegar is used as food preservative- vinegar is acetic
acid)
(iv) Calculation of equilibrium constant (Keq)
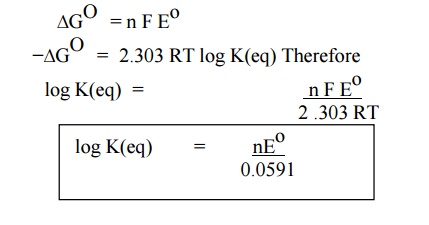
(v) Calculation of Standard emf of the cell
E cell = E cathode − E anode
( if both
reduction potentials are considered)
E cell = E cathode + E anode
( if
oxidation potential of anode and the reduction potential of cathode are
considered)
Corrosion
The metals higher in the series are anodic and are
more prone to corrosion.
The metals lower in the series are noble metals
(cathodic) and they are less prone to corrosion.
Predicting
the spontaneity of cell reaction
Spontaneity of the redox reaction can be predicted
from the emf value of complete cell reaction.
If the value of Ecell is positive, the reaction is feasible. as ∆G
will be negative
( i.e. it
is an electrochemical cell)
If the value of Ecell is negative, the reaction is not feasible.
as ∆G will be positive
( i.e. it
is an electrolytic cell)
6 NERNST EQUATION
If an emf
of a reversibile cell is E volts and the cell reaction that takes place is
accocated with the passage of an faradays or nF coulombs, the electrical work
done by the system is given by the product of the emf and total charge.
Electrical
work done by the system = nFE, where E is the reversibile emf of the cell and n
is the no.of electrons involved in the cell reaction.
ΔThe work done on the system at constant temperature is the increase in the free energy ΔG of the system.
The free
energy change of a reaction depends on the activities of the reactants and the
products. Therfore, in the case of cell reactions of a reversibile, the emf of
the cell will also depend on the activities of the reactant and products.
Applications of Nernst Equation :
I)To
calculate electrode potential of unknown metal. ii)To predict corrosion of
metals.
iii) To
set up electrochemical series.
6.1Nernst equation for a reversible cell :
Let us
consider the reaction in a reversible cell :
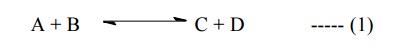
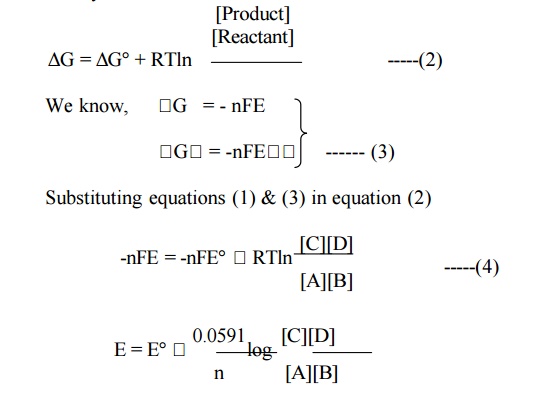
This is
Nernst equation for a reversible cell.
6.2 PROBLEM BASED ON NERNST
EQUATION
Calculate the reduction potential of lead electrode
in contact with a solution of 0.015M Pb2+ ions.( E0 =
-0.13 volt)
GIVEN
Std
Oxidation potential is given as Pb2+ + 2 e- → Pb; E0
= -0.13v
Concentration of Pb2+ = 0.015M
Solution
The
Nernst equation for reduction potential is
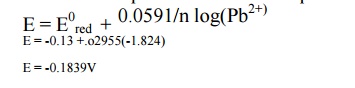
Oxidation
potential of Pb = -0.1839V
Related Topics