Chapter: Chemistry : Water Technology
Important Questions and Answers: Water Technology
01. Define boiler feed water.
The water
fed into the boiler for the production of steam is known as boiler feed water.
02. What are the requirements of boiler feed water?
It should
free from
(i).
Suspended solids. (ii). Dissolved salts like MgCl2
(iii).
Hardness. (iv). Alkalinity. (v). Dissolved gases like O2 and CO2
03. What are the disadvantages using hard water in
boiler?
Scale and
sludge formation. 2. Caustic
Embrittlement.
3.
Priming and Foaming. 4. Boiler
corrosion.
04. What are the disadvantages formation of
deposits in steam boilers and heat exchangers?
Wastage
of fuels. 2. Decrease in efficiency. 3. Boiler explosion.
05. Define Boiler explosion.
When
thick scales crack due to uneven expansion, the water comes suddenly in contact
with over-heated iron plates. This causes in formation of a large amount of
steam suddenly. So sudden high-pressure is developed, which may even cause
explosion of the boiler.
06. What are the methods used to be prevention of
scale formation?
Prevention
of scales formation: (i).
External Treatment – zeolite process
(ii).
Internal Treatment.- carbonate conditioning.
07. What are the methods used to be softening hard
water?
(i).
External Treatment – zeolite process, Demineralisation process (ii). Internal
Treatment.- carbonate conditioning, calgon conditioning.
08. What is meant by internal conditioning of
water? Give one example.
Treating
the boiler water in the boiler itself by adding chemicals to remove scale
forming substance is called internal conditioning.
Ex:
carbonate conditioning, phosphate conditioning, calgon conditioning.
09. What is meant by external conditioning of
water? Give one example.
Treating
the boiler water before feeding it into boiler is called external conditioning.
Ex: zeolite process, Demineralisation process
10. Distinguish between internal contioning and
external conditioning methods.
Internal contioning
To remove hardness producing salts, chemicals were added to the
boiler water in the boiler itself and that treatment is known as internal
conditioning.
External conditioning
Treating the boiler water before feeding it into boiler is
called external contioning.
11. What is meant by caustic embrittlement? How is
it prevented?
Caustic
embrittlement means intercrystalline cracking of boiler metal. It is prevented
by using softening agent like sodium phosphate and by adding tannin and lignin.
12. What is desalination?
The
process of removing common salt (NaCl) from the water is known as desalination.
The water contains dissolved salts with brackish taste is called brackish
water.
Desalination
= Removal of common salt (NaCl ) from water
13. What are boiler compounds? Mention two
different boiler compounds and their actions.
The
chemicals directly added into the boiler for removing scale forming substances
is known as boiler compounds. Ex: Sodium carbonate and Sodium phosphate
14. What is Calgon? How does it function in water
treatment?
Calgon –
Sodium Hexa Meta Phosphate
It
interacts with calcium ions forming a highly soluble complex and thus prevents
the precipitation of scale forming salt.
15. What is Reverse osmosis?
When two
solutions of different concentrations are separated by a semipermeable
membrane, when a pressure is applied on the concentrated side, the solvent flow
from higher concentration to lower concentration.
16. What are the advantages of reverse osmosis method?
Low
capital cost, easy operating.
RO method
is used for converting sea water into drinking water.
It
removes all types of impurities like non-ionic and colloidal.
The life
time of membrane is high and it can be replaced within few minutes.
01. Define Boiler Feed Water. What are the
requirements of Boiler Feed Water?
Boiler Feed Water: The
water fed into boiler for the production of steam is called boiler feed water.
It should
be free from turbidity, oil, dissolved gases, alkali and hardness producing
substances.
Requirements
of Boiler Feed Water:
i). It
should has zero hardness.
If
hardness present in boiler feed water, it produces scales and sludges, which
prevents efficient heat transfer.
ii). It
must free from dissolved gases like O2 and CO2.
![]()
If
dissolved gases present in boiler feed water, it leads to boiler corrosion.
iii). It
should be free from suspended impurities.
If it is
present in boiler feed water, it produces wet steam.
iv). It
should be free from dissolved salts and alkalinity.
If it is
present in boiler feed water, it produces caustic embrittlement, which causes
brittlement of boiler parts.
Zeolite:Sodium
Aluminum Orthosilicate.
Na2O.Al2O3.xSiO2YH2O.
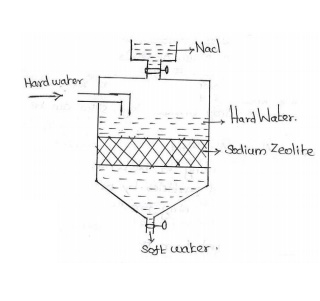
The
synthetic form of zeolite is known as PERMUTIT, which is porous and possess gel
structure and Ze stands for insoluble zeolite.
Principle:
The sodium ions which are loosely held in Na2Ze are replaced by Ca2+ and Mg2+
ions present in water.
Process: When
hard water is passed through a bed of zeolite placed in a closed cylinder, the
hardness causing ions like Ca2+ and Mg2+ ions are taken
up by zeolite. Sodium salts are released during the reaction as byproduct.
For softening of water by zeolite process, hard water is percolated at a
specified rate through a bed of zeolite, kept in a cylinder.
The hardness causing ions like Ca2+ and Mg2+are
retained by the zeolite as CaZe and MgZe. While the outgoing water contains
sodium salts.
The
various reactions taking place may be
Na2Ze
+ Ca(HCO)2 -- --- > CaZe + 2NaHCO3
Na2Ze
+ Mg(HCO)2 -- --- > MgZe +
2NaHCO3
Na2Ze + MgCl2 -- --- > MgZe +
2NaCl
![]() Na2Ze + CaCl2 -- --- > CaZe + 2NaCl
Na2Ze + CaCl2 -- --- > CaZe + 2NaCl
Na2Ze
+ CaSO4 -- --- > CaZe
+ Na 2SO4
Na2Ze
+ MgSO4 -- --- > MgZe
+ Na 2SO4
Regeration
After some time zeolite gets exhausted. The exhausted zeolite is
again regerated by treating with 10%solution of NaCl.
CaZe + 2 NaCl → Na2Ze + CaCl2
MgZe + 2 NaCl → Na2Ze
+MgCl2
Advantages: (i). Output water has only 1-2 ppm.
(ii). Operation is easy.
(iii). No
sludge is formed during the process.
03. Explain formation of deposits
in steam boilers and heat exchangers.
Steamboilers: Sealed
vessel where water is converted to steam.
A steam
boiler is a type of generator that is used to create steam.
Heat
exchangers: A device for transferring heat from one medium to
another.
Heat
exchangers are designed to remove excess heat from aircraft
engines, optics, x-ray tubes, lasers, power supplies, military equipment, and many other types of equipment
that require cooling beyond what air-cooled heat sinks can provide.
Formation
of deposits: Scale and sludge formation.
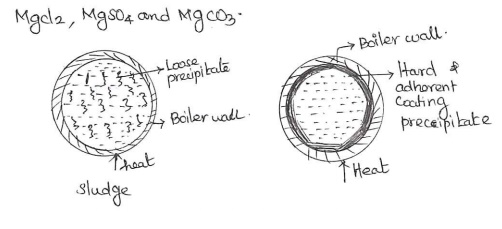
Scale:If the
precipitate forms hard and adherent coating on the inner walls of the boiler is
known as scale. It is formed by the
substances like CaSO4, Mg(OH)2 and Ca(HCO3)2.
Scales
are difficult to remove even with the help of hammer and chisel. Scales are the
main sources of boiler troubles. Formation
of scales
may be due to
(i).
Decomposition of calcium bicarbonate. (ii). Deposition of calcium sulphate.
(iii).
Hydrolysis of magnesium salts. (iv). Presence of silica.
Sludge: In boiler, water contains the
precipitate loose and slimy is known as sludge. It is formed by the substances like CaCl2, MgCl2, MgSO4 and MgCO3.
Disadvantages:
(i). Poor
conductor of heat.
(ii).
Excessive sludge formation disturbs the working of the boiler.
(iii). It
forms along with scales, then former gets entrapped in the latter and both get
deposited scales.
Prevention
of sludge formation: (i). By using well softened water.
(ii). By
frequently below down opearation.
04. What are the disadvantages in
scale formation? Explain in detail.(Or)
What are the disadvantages formation
of deposits in steam boilers and heat exchangers?(or)
Write short notes on (i). Wastage
of fuels. (ii). Decrease in efficiency. (iii). Boiler Explosion.
Disadvantages: (i).
Wastage of fuels.
(ii).
Decrease in
efficiency.
(iii). Boiler
Explosion.
(i). Wastage of fuels:
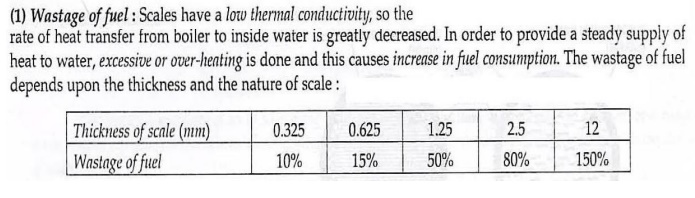
(ii). Decrease in efficiency:
Scales may sometimes deposit in the valves and
condensers of the boiler and choke then partially. This results in decrease in
efficiency of the boiler.
(iii). Boiler Explosion:
When thick scales crack due to uneven expansion,
the water comes suddenly in contact with over-heated iron plates. This causes
in formation of a large amount of steam suddenly. So sudden high-pressure is
developed, which may even cause explosion of the boiler.
6. Explain prevention of scales
formation.
Prevention of scales formation: (i). External Treatment.
(ii).
Internal Treatment.
(i).
External Treatment:Softening of water (i.e) removing hardness-
producing constituents of water. Ex: Zeolite process and Demineralization
process.
(ii).
Internal Treatment:Sequestration
process.
An ion is prohibited to exhibit its original
character by complexing or converting it into other more soluble salt by adding
suitable reagent.
An
internal treatment – by adding proper chemical to the boiler water either
(a). To
precipitate the scale forming impurities in the form of sludge, which can be
remove by blow – down operation (or)
(b). To
convert them into compounds, which will stay in dissolved form in water and
thus do not cause any harm Blow down operation: It is partial removal of hard
water through top at the bottom of boiler, when extent of hardness in the
boiler becomes alarmingly high.
Internal
treatments methods are, generally, followed by blow-down operation , so that
accumulated sludge is removed. Internal treatment methods are,
(i).
Colloidal Conditioning
(ii).
Phosphate Conditioning
(iii).
Carbonate Conditioning
(iv).
Calgon Conditioning.
07. Write short notes on Caustic Embrittlement.
Caustic
Embrittlement. = Intercrystalline cracking of boiler metal
It is a
type of boiler corrosion, caused by using highly alkaline water in the boiler.
In boiler
water, it contains a small amount of sodium Carbonate. In high pressure, it
decomposes to give sodium hydroxide.
Na2CO3 + H2O
--- 2NaOH + CO2
Then this
NaOH reacts with boiler material and it forms sodium Ferrate and this leads to
brittlement of boiler parts like joints, bends etc.,
Fe +2NaOH --- Na2FeO2
+ H2
It is prevented by or
Avoided by
(i). By
using sodium phosphate as softening reagent instead of sodium carbonate. (ii).
By adding Tannin or Lignin to boiler water for blocks hair-cracks.
(iii). By
adding sodium sulphate to boiler water also blocks hair-cracks.
08. Explain Internal conditioning
methods of softening hard water.
To remove
hardness producing salts, chemicals were added to the boiler water in the
boiler itself and
that
treatment is called internal conditioning.
(i).
Colloidal Conditioning (ii). Phosphate Conditioning
(iii).
Carbonate Conditioning (iv). Calgon Conditioning.
(i)
Colloidal Conditioning:
In low-pressure
boilers, scale formation
can be avoided
by adding organic
substances like kerosene ,tannin,
agar-agar (a gel), etc., which get coated over the scale forming precipitates,
thereby yielding non-sticky and loose deposits, which can easily be removed by
pre-determined blow-down operations.
(ii). Phosphate
conditioning:
Three types
of phosphates- mono, di and trisodium
phosphates are employed
in phosphate conditioning. The advantages of phosphate conditioning over
carborate conditioning are
(i). It
can be applied to high pressure boilers and
(ii) It
can be used for softening/ conditioning acidic, neutral or alkaline water
sample.
If acidic
water is to be conditioned, trisodium phosphate can be used. For neutral and
alkaline water samples disodium phosphate and monosodium phosphate can be used
respectively.
(iii)
Carbonate conditioning:
In low
pressure boilers, calcium ions are converted into soft and loose sludge by
adding sodium carbonate solution. It forms soft CaCO3 which can be removed by
blow-down operation.
Calgon
conditioning:
Calgon
interacts with calcium ions forming a highly soluble complex and it prevents
the
Calgon=Sodium HexaMeta phospha)6precipitation of scale forming salt. The complex Na2[Na4(PO3)6]
is soluble in water and no problem for its sludge disposal.
Write short
notes on Boiler corrosion / Explain boiler corrosion in detail / Write short
notes on Boiler troubles – Boiler corrosion.
Boiler
corrosion taken place in boiler in the presenceë of gases like dissolved oxygen,û dissolved CO2 and dissolved salts.
Dissolved oxygen: It
attacks the boiler material at high temperature and causes the Corrosion.
4Fe +6H2O + 3O2
--- > 4Fe(OH)3
It is removed by chemical and mechanical method. Chemical
Method: Sodium sulphite, Hydrazine used to remove dissolved oxygen. Mechanical
Method: To remove by De-aeration method.
Water
spraying in a perforated plate-fitted tower, heated from sides and connected to
vaccum pump. High temperature, low pressure and large exposed surface reduces
the dissolved oxygen in water.
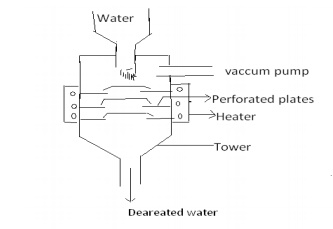
Dissolved CO2: It produces carbonic acid, which is acidic
and corrosive in nature
CO2 +H2O
--- H2CO3 (Carbonic acid )
It can be
removed by adding calculated amount of NH4OH into water and also removed by
de-aeration method.
Dissolved salts(MgCl2): Salts
like CaCl2 and MgCl2 undergoes hydrolysis at higher temperature , to give
HCl , which corrodes the boiler
MgCl2
+ 2H2O --- Mg(OH)2 +2HCl
Fe +2HCl ---
FeCl2 + H2
FeCl2 + 2H2O
---- Fe(OH)2 +2HCl
It can be
avoided by adding alkali into the boiler water.
10. Explain priming and foaming
(carry over).
1.Priming: It
is the process of production of wet steam.
Wet steam
– Steam contains droplets of liquid water
It is
caused by
High
steam velocity
Very high level water in the boiler
Sudden boiling of water
iv).Very
poor boiler design.
Prevention: It is controlled by
Controlling the velocity of steam
Keeping the water level lower
Good boiler design
4) Using
treated water
2)
Foaming:
The
formation of stable bubbles above the surface of water is called foaming
These
bubbles are carried over by steam leading to excessive priming. It is caused by
Presence
of oil and grease
Presence of finely divided particles. It can be
prevented by
i).
Adding coagulants like sodium aluminate
ii).Adding
anti-foaming agents like synthetic polyamides.
11. What is Desalination? Describe desalination of
by Reverse Osmosis method with neat diagram.(or)Explain the reverse osmosis
process for desalination of brackish water in detail.
Desalination
= Removal of common salt-NaCl from
water.
Brackish
water = Water containing dissolved salts with a peculiar salty taste. Ex: Sea
water
Reverse
Osmosis(R
O):
When a
pressure greater than osmotic pressure applied on the concentrated side, the
solvent flow takes place from higher concentration to lower concentration is
known as reverse osmosis.
In this RO process, Pure water is separated from
salt water.
This RO process is also known as Super filtration
or Hyper filtration
When the pressure is applied from the higher
concentration side, the solvent flow takes place to lower side and these two
concentrations are separated by semipermeable membrane, the salt water is
converted into pure water.
The membranes used as cellulose acetate, polyamide
and some polymers.
Advantages &
RO method:
1. Low capital cost, easy operating.
2. It is
used for converting sea water into drinking water
3. It
removes all types of impurities like non-ionic and colloidal
4. The life
time of membrane is high and it can be replaced within few minutes.
Related Topics