Chapter: Chemistry : Water Technology
Water Technology
WATER TECHNOLOGY
1 Introduction
2 Hard water and soft water
3 Boiler Feed water
3.1 Formation of deposits in steam boilers and Heat
exchangers
3.2 Caustic embrittlement
3.3 Boiler corrosion
3.4 Priming and Foaming
4 Softening or conditioning Methods
4.1 Zeolite or Permutit process
4.2 Ion-Exchange (or) De- ionization (or)
Demineralisation Process
5 Internal Treatment (or) Internal Conditioning
(or) Boiler Compounds
6 Desalination of Brackish water
1 INTRODUCTION
Water is essential for the existence
of human beings, animals and plants. Though 80% of the earth‟s surface is
occupied by water, less than 1% of the water is available for ready use.
The main sources of water are
Rain
rivers and lakes (surface water)
wells and springs (ground water)
sea water
Among the above sources of water, rain water is the purest form
of water but it is very difficult to collect whereas sea water is the most
impure form.
Thus, surface and ground water are
normally used for industrial and domestic purposes. Such water must be free
from undesirable impurities.
“The process of removing all types of
impurities from water and making it fit for domestic or industrial purposes are
called water treatment.” Before treating
water one must know the nature as well as the amount of impurities.
2 HARD WATER AND
SOFT WATER
Hard Water
“Water which does not produce lather with soap solution, but
produces white precipitate is called hard water”.
This is due to the presence of dissolved Ca and Mg salts.
Soft Water
“Water which produces lather readily with soap solution is
called soft water.”
This is due to the absence of Ca and Mg salts.
3 BOILER FEED WATER
In Industry, one of the chief uses of
water is generation of steam by boilers. The water fed into the boiler for the
production of steam is called boiler feed water.
Requirements of
boiler feed water
It should have zero hardness.
It must be free from dissolved gases
like O2, CO2, etc.
It should be free from suspended
impurities.
It should be free from dissolved
salts and alkalinity
It should be free from turbidity and
oil.
It should be free from hardness
causing and scale forming constituents like Ca and Mg salts.
Formation of deposits (Scales and
Sludges) in boilers and heat exchangers
In a boiler, water is continuously converted into steam. Due to
this continuous evaporation of water, the concentration of soluble matters
increases progressively. Then the salts separating out from the solution in the
order of their solubility, the lease soluble ones separating out first.
(i) Sludge
If the precipitate is loose and slimy it is called sludges.
Sludges are formed by substances like MgCl2, MgCO3,
MgSO4 and CaCl2.
They have greater solubilities in hot water than cold water.
( i i ) Scale
If the precipitate forms
hard and adherent coating on the inner walls of the boiler, it is called scale.
Scales are formed by substances like Ca (HCO3)2,
CaSO4 and Mg(OH) 2.
Disadvantages of Scale Formation
Wastage of fuels
Scales have a low thermal conductivity, so the rate of heat
transfer from boiler to inside water is greatly decreased. In order to provide
a supply of heat to water, excessive or over-heating is done. This causes
increase in fuel consumption. The wastage of fuel depends upon the thickness
and the nature of scale.
(ii) Decrease in efficiency
Scales sometimes deposit in the valves and condensers of the
boiler and choke them partially. This results in decrease in efficiency of the
boiler.
(iii) Boiler explosion
When thick scales crack due to uneven expansion, the water comes
suddenly in contact with over-heated iron plates. This causes in formation of a
large amount of steam suddenly. So sudden high-pressure is developed, which may
even cause explosion of the boiler.
Prevention of scale formation
At the initial stage, scales can be
removed using scraper, wire brush etc.
If scales are brittle, they can be
removed by thermal shocks.
If the scales are loosely adhering,
they can be removed by frequent blow down operation.
Caustic Embrittlement
Caustic embrittlement is a form of corrosion caused by a high
concentration of sodium
Hydroxide in the boiler feed water.
It is characterized by the formation of irregular
intercrystalline cracks on the boiler metal, particularly at places of high
local stress such as bends and joints.
Causes of caustic embrittlement
Boiler water usually contains a small
amount of Na2CO3. In high pressure boilers, Na2CO3
undergoes hydrolysis to produce NaOH.
Na 2 CO 3 + H 2 O
→ 2NaOH +CO2
This NaOH flows into the minute
hairline cracks present on the boiler material by capillary action and
dissolves the surrounding area of iron as sodium ferroate, Na2FeO2.
Fe + 2NaOH →
Na 2 FeO 2+H2
This type of
electrochemical corrosion occurs
when the concentration
of NaOH is above 100 ppm. This causes embrittlement of boiler parts,
particularly the stressed parts like bends, joints, rivets, etc.
Caustic embrittlement can be prevented by
Using sodium phosphate as the softening agent instead of sodium carbonate.
Adding chemicals such as tannin, lignin to the boiler water.
They block the hairline cracks.
Adjusting the pH of the feed water carefully between 8 and 9.
Boiler Corrosion
Corrosion in boilers is due to the presence of
Dissolved oxygen
Dissolved carbon dioxide
Dissolved salts like magnesium
chloride.
Dissolved oxygen
The presence of dissolved oxygen is
responsible for corrosion in boilers. Water containing dissolved oxygen when
heated in a boiler, free oxygen is evolved, which corrodes the boiler material.
4Fe + 6H2O +
3O2 → 4
Fe(OH)3
Dissolved carbon dioxide
When water containing bicarbonates is
heated, carbon dioxide is evolved which makes the water acidic. Carbon dioxide
dissolved in water forms carbonic acid. This leads to intense local corrosion
called pitting corrosion.
Ca(HCO3)2 → CaCO3
+ H2O + CO2
CO2 + H2O → H2CO3
Dissolved magnesium chloride
When water containing dissolved
magnesium chloride is used in a boiler, hydrochloric acid is produced. HCl
attacks the boiler in a chain-like reaction producing hydrochloric acid again
and again which corrodes boiler severely.
MgCl2 + 2H2O → 2HCl + Mg (OH)2
Fe + 2 HCl → FeCl2
+ H2
FeCl2 + 2H2O → Fe (OH)2
+ 2 HCl
Corrosion by HCl can be avoided by the addition of alkali to the
boiler water.
Prevention of boiler corrosion
Removal of dissolved oxygen and
carbon dioxide can be done either chemically or mechanically.
Chemical method
For the removal of dissolved oxygen, sodium sulphite, hydrazines
are used.
2Na2SO3 + O2 → 2Na2SO4
N2H4 + O2 →
N2 +
2H2O
Hydrazine is the ideal compound for
the removal of dissolved O2 as it forms only water and inert
nitrogen gas during the reaction.
Dissolved CO2 is removed
by the addition of ammonium hydroxide. 2NH4OH + CO2 → (NH4)2CO3 + H2O
Mechanical method
Oxygen along with carbon dioxide can
be removed mechanically by the de-aeration method
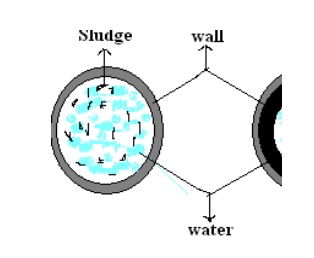
In this method, water is allowed to
fill in slowly on the perforated plates fitted inside the tower.
To reduce the pressure inside the
tower, the de-aerator is connected to a vacuum pump. The sides of the tower are
heated by means of a steam jacket. This is based on the principle that the
solubility of a gas in water is directly proportional to pressure and inversely
proportional to temperature.
High temperature, low pressure and a
large exposed surface, reduces the dissolved gases (O2 and CO 2)
in water.
The water flows down through a number
of perforated plates and this arrangement exposes a large surface of water for
de-aeration.
Priming and Foaming
During the production of steam in the
boiler, due to rapid boiling, some droplets of liquid water are carried along
with steam. Steam containing droplets of liquid water is called wet steam.
These droplets of liquid water carry
with them some dissolved salts and suspended impurities. This phenomenon is
called carry over. It occurs due to priming and foaming.
Priming
Priming is the process of production of wet steam. Priming is
caused by
High steam velocity.
Very high water level in the boiler.
Sudden boiling of water.
Very poor boiler design.
Prevention
Priming can be controlled by
Controlling the velocity of steam.
Keeping the water level lower.
Good boiler design.
Using treated water.
Foaming
The formation of stable bubbles above
the surface of water is called foaming. These bubbles are carried over by steam
leading to excessive priming.
Foaming is caused by the
Presence of oil and grease.
Presence of finely divided particles.
Prevention
Foaming can be prevented by
Adding coagulants like sodium
aluminate, aluminium hydroxide.
Adding anti-foaming agents like
synthetic polyamides.
4 PREVENTION OF
SCALE FORMATION (OR) SOFTENING OF HARD WATER
The process of removing hardness –
producing salts from water is known as softening or conditioning of water.
Since water is a source for industrial purpose. It is mandatory to soften water
to make it free from hardness producing substances, suspended impurities and
dissolved gases, etc.
Softening of water can be done by two methods.
i) External treatment
ii) Internal treatment.
External Treatment
or Conditioning
It involves the removal of hardness producing salts from the
water before feeding into the boiler. The external treatment can be done by the
following methods.
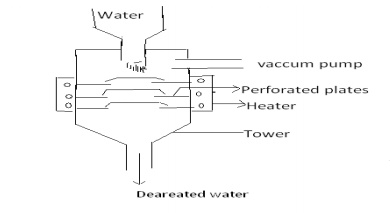
Zeolite (or) Permutit process
1 Zeolite (or)
Permutit process
Zeolites are naturally occuring
hydrated sodium aluminosilicate minerals. The chemical formula is Na2O.Al2O3.XSiO2.YH2O.
The synthetic form of zeolite is called permutit and is represented by Na2Ze.
In this process the hard water is
allowed to perlocate through sodium zeolite. The sodium ions which are loosely
held in this compound are replaced by Ca2+ and Mg2+ ions.
When zeolite comes in contact with
hard water, it exchanges its sodium ions with calcium and magnesium ions of
hard water to form calcium and magnesium zeolites.
As sodium ions do not give any
hardness to water, the effluent will be soft. The exhausted zeolite is again
regenerated by treated with 5 to 10 percent of sodium chloride solution.
Na2Ze
+ Ca(HCO)2 -- -- >
CaZe + 2NaHCO3
Na2Ze
+ Mg(HCO)2 -- -- > MgZe
+ 2NaHCO3
Na2Ze
+ MgCl2 -- -- >
MgZe
+ 2NaCl
Na2Ze
+ CaCl2 -- -- >
CaZe + 2NaCl
Na2Ze
+ CaSO4 -- -- >
CaZe + Na 2SO4
Na2Ze
+ MgSO4 -- -- >
MgZe
+ Na 2SO4
Regeration
After some time zeolite gets exhausted. The exhausted zeolite is
again regerated by treating with 10%solution of NaCl.
CaZe + 2 NaCl → Na2Ze + CaCl2
MgZe + 2 NaCl → Na2Ze
+MgCl2
Advantages
No sludge is formed during this
process.
Water of nearly zero hardness is
obtained.
This method is very cheap because the
generated permutit can be used again.
The equipment used is compact and
occupies a small space.
Its operation is also easy.
The process can be made automatic and
continuous.
Disadvantages
This process cannot be used for
turbid and acidic water as they will destroy the zeolite bed.
This treatment replaces only the
cations, leaving all the anions like
(HCO3)–
and(CO3)2– in the soft water.
· When such water is
boiled in boilers, CO2 is liberated. Free CO2 is weakly
acidic in nature and extremely corrosive to boiler metal.
Na2CO3 + H2O→2NaOH + CO2
Due to the formation of sodium
hydroxide, the water becomes alkaline and can cause cause caustic
embrittlement.
Water containing Fe, Mn cannot be
treated, because regeneration is very difficult.
This process cannot be used for
softening brackish water. Because brackish water also contains Na+ ions. So, the ions
exchange reaction will not take place.
2 Ion exchange or Demineralisation
process
Ion exchange or demineralisation
process removes almost all the ions (both anions and cations) present in the
hard water.
The soft water, produced by lime-soda
and zeolite processes, does not contain hardness producing Ca2+ and
Mg2+ ions, but it will contain other ions like Na+, K+,
SO42– , Cl– etc.,
On the other hand demineralised (DM)
water does not contain both anions and cations. Thus a soft water is not
demineralised water whereas demineralised water is soft water.
This process is carried out by using
ion exchange resins, which are long chain, cross linked, insoluble organic
polymers with a micro process structure. The functional groups attached to the
chains are responsible for the ion exchanging properties.
(i) Cation exchanger
Resins containing acidic functional groups
(–COOH, – SO3H) are capable
of exchanging their H+ ions with other cations of hard water.
Cation exchange resin is represented as RH2.
Examples:
Sulphonated coals
Sulphonated polystyrene R–SO3H;
R–COOH ≡ RH2
Anion Exchanger
Resins containing basic functional
groups (–NH2, –OH) are capable of exchanging their anions with other
anions of hard water.
Anion exchange resin is represented as R (OH)2.
Examples:
· Cross-linked
quaternary ammonium salts. Urea-formaldehyde resin.
R–NR3OH; R–OH; R–NH2 ≡ R (OH)2
Process
The hard water first passed through a
cation exchange which absorbs all the cations like Ca2+, Mg2+
Na+, K+, etc. present in the hard water.
RH2 + CaCl2 → RCa + 2HCl
RH2 + MgSO4 → RMg + H2SO4
RH + NaCl → RNa + HCl
The cation free water is then passed
through a anion exchange column, which absorbs all the anions like Cl–,
SO42, HCO3–, etc., present in the
water.
R' (OH) 2 + 2HCl → R'Cl2 + 2H2O R'(OH) 2 + H2SO4
→ R'SO4 + 2H2O
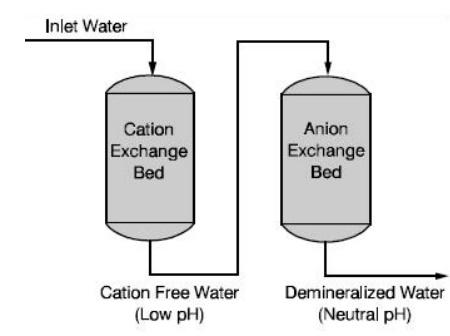
The water coming out of the anion exchanger completely free from
cations and anions. This water is known as demineralised water or deionised
water.
Regeneration
When the cation exchange resin in
exhausted, it can be regenerated by passing a solution of dil.HCl or dil.H2SO4.
RCa + 2HCl → RH2
+ CaCl2
RNa + HCl → RH + NaCl
Similarly, when the anion exchange
resin is exhausted, it can be regenerated by passing a solution of dil.NaOH.
R'Cl2 + 2 NaOH →
R'(OH)2 +
2 NaCl
Advantages
The water is obtained by this process will have very low
hardness.
Highly acidic or alkaline water can be treated by this process.
Disadvantages
The equipment is costly.
More explosive chemicals are needed for this process.
Water containing turbidity, Fe and Mn cannot be treated, because
turbidity reduces the output and Fe, Mn form stable compound with the resins
.
5 INTERNAL TREATMENT
Internal treatment involves adding chemicals directly to the
water in the boilers for removing dangerous scale – forming salts which were
not completely removed by the external treatment for water softening. This
method is used to convert scale to sludge which can be removed by blow-down
operation.
Calgon conditioning
Carbonate conditioning
Phosphate conditioning
Colloidal conditioning
Calgon conditioning
Calgon is sodium hexa meta phosphate with a
Composition Na2(Na4 (PO3)6).
A highly soluble complex containing Ca is formed by replacing the sodium ions
and thus prevents their formation of scale forming salts like CaSO4.
The reaction is as follows:
2CaSO 4 + Na 2 [Na 4
(PO3) 6] → Na 2 [Ca 2 (PO3)
6] + 2Na 2SO4
Since the complex is highly soluble there is no problem of
sludge disposal.
Carbonate conditioning
Scale formation due to CaSO4
in low pressure boilers can be avoided by adding Na2CO3
to the boilers.
CaSO4 + Na2 CO3 → CaCO3
+ Na2SO4
The forward reaction is favored by
increasing the concentration of CO32-.CaCO3 formed can be removed easily.
Phosphate
conditioning
In high pressure boilers, CaSO4
scale whose solubility decrease with increase of temperature. Such scale can be
converted into soft sludge by adding excess of soluble phosphates.
3CaSO4 + 2Na3 PO4 → Ca3
(PO4)2 +2Na2SO4
There are three types of phosphates employed for this purpose.
Tri-sodium phosphate – Na3PO4 (too
alkaline): used for too acidic water.
Di-sodium hydrogen phosphate – Na2HPO4
(weakly alkaline): Used for weakly acidic water.Mono sodium di hydrogen
phosphate NaH2PO4 (acidic) used for alkaline acidic
water.
Colloidal
conditioning
The colloidal conditioning agents are kerosene, agar-agar,
gelatin, glue, etc. They are Used in low pressure boilers. The colloidal
substances convert scale forming substance like CaCO3, CaSO4
into a Non-adherent, loose precipitate called sludge, which can be removed by
blow-down Operation.
6 DESALINATION OF
BRACKISH WATER
Depending upon the quantity of dissolved solids, water is graded
as Fresh water has < 1000 ppm of dissolved solids. Brackish water has >
1000 but < 35,000 ppm of Dissolved solids.
Sea water has > 35,000 ppm of dissolved solids.
Water containing dissolved salts with a peculiar
salty or brackish taste is called brackish water. It is totally unfit
for drinking purpose. Sea water and brackish water can be made available as
drinking water through desalination process.
The removal of dissolved solids
(NaCl) from water is known as desalination
process. The need for such a method arises due to the non-availability of fresh water. Desalination is carried
out either by electro dialysis or by reverse osmosis.
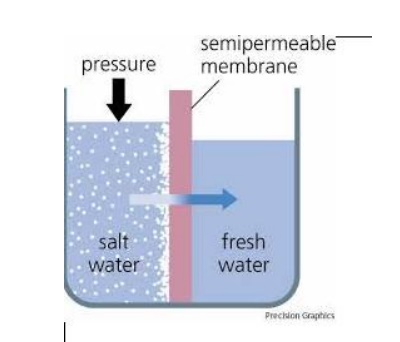
Reverse Osmosis
When two solutions of different
concentrations are separated by a semi-permeable membrane, flow of solvent
takes place from a region of low concentration to high concentration until the
concentration is equal on both the sides. This process is called osmosis.
The driving forces in this phenomenon are called osmotic pressure. If a hydrostatic pressure in excess of osmotic pressure is applied on the higher concentration side, the solvent flow reverses, i.e., solvent is forced to move from higher concentration to lower concentration .This is the principle of reverse osmosis. Thus, in reverse osmosis method pure water is separated from its dissolved solids.
sing this method pure water is separated from sea water. This process is also known as super-titration. The membranes used are cellulose acetate, cellulose butyrate, etc.
Advantages
The life time of the membrance is high.
It can be replaced within few minutes.
It removes ionic as well as non-ionic, colloidal impurities
Due to simplicity low
capital cost, low operating, this process is used for converting sea water into
drinking water
Glossary
Hardness
Harness is the property or
characteristics of water, which does not produce lather with soap solution.
Soft water
Water
which produces lather readily with soap solution is called soft water. Soft water is free of calcium & magnesium salts.
Temporary hardness
Temporary hardness is due to
the presence of bicarbonates of calcium and
magnesium. Since these salts can be easily removed by simple physical
methods such as boiling and filtering.
Permanent hardness
Permanent
hardness is due to the presence of soluble chlorides and sulphates of calcium
and magnesium. These salts can be removed by chemical treatments only.
Alkalinity
Alkalinity
of water is due to the presence of soluble hydroxide ( OH- ),
carbonate ( CO32-) and bicarbonate ( HCO3-) ions.
Boiler feed water
The water
which is free from dissolved salts, dissolved gases, hardness, oils and
alkalinity is known as boiler feed water.
Sludge
The loose
and slimy precipitate is called sludge.
Scale
The hard
and adherent precipitate on the inner walls of the boiler is called scale.
Priming
Some
droplets of liquid water are carried along with steam during the production of
steam in the boiler is called priming.
Foaming
The
formation of stable bubbles above the surface of water is called foaming.
Caustic embrittlement
Formation
of irregular, intergranular cracks at the welded joints, rivets etc. in high
pressure boilers is called caustic embrittlement.
Brackish water
The water
containing high concentration of dissolved salts with salty or brackish taste
is called brackish water.
Reverse osmosis
The
solvent flows from higher concentration to lower concentration.
Related Topics