Chapter: Chemistry : Phase Rule and Alloys
Phase Rule and Alloys
PHASE RULE AND ALLOYS
Chemical reactions are of two types
1.Irreversible
reaction
2. Reversible reaction – it’s of two types
a. homogeneous
b. heterogeneous
A.
homogeneous reversible reaction
B.heterogeneous reversible reaction
--- its behaviour can be studied by PHASE
RULE given by Willard Gibbs (1874).
Phase rule
The
number of degree of freedom (F) of the system is related to number of
components (C) and number of phases (P) by the following phase rule equation.
F = C-P+2
Explanation or meaning of terms 1. Phase
(P)
Any
homogeneous physically distinct and mechanically separable portion of a system
which is separated from other parts of the system by definite boundaries.
a.
Gaseous phase
All gases are completely miscible and there is
no boundary between one gas and the other. For example: air – single phase
b.Liquid
phase
It depends on the number of liquids present and
their miscibilities.
i.
If two
liquids are immiscible, they will form three separate phases two liquid phase
and one vapour phase. For example: benzene-water.
ii.
If tow
liquids are miscible, they will form one liquid phase and one vapour phase. For
example: alcohol – water.
C .Solid
phase
Every solid constitutes a separate phase
For
example:
(i)
Water system ------- three phases
(ii)
Rhombic sulphur (s) -- > monoclinic sulphur (s) ----- two phase
iii)
Sugar solution in water ----- one phase
iv) CuSO4.5H2O(s) < -- -- > CuSO4.3H2O(s)
+ 2H2O(g) ---- three phases.
2.
Component (C)
“The
smallest number of independently variable constituents, by means of which the omposition
of each phase can be expressed in the form of a chemical equation”.
For
example:
i) Water system ---- one component ( H2O )
ii) An aqueous system of NaCl --- two
component ( NaCl , H2O )
iii) PCl5(s) < -- -- > PCl3
(l) + Cl2 (g) --- two
component ,three phases
iv) CuSO4.5H2O(s) < -- -- > CuSO4.3H2O(s) + 2H2O(g) ---- three phases,two
3. Degree
of freedom(F)
“The minimum number of independent variable factors such as
temperature, pressure and concentration, which much be fixed in order to define
the system completely”.
i) Water system
Ice (s)
< -- -- > water (l) < -- -- > vapour (g)
F = Non
variant (or) zero variant
ii) Ice (s) < -- -- > water (l)
F =
univariant (one)
iii) For
a gaseous mixture of N2 and H2, we must state both the pressure and
temperature.
Hence,the
system is bivariant.
PHASE DIAGRAM:
Phase diagram is a graph obtained by plotting
one degree of freedom against another.
Types of phase diagrams
(i)P-T
Diagram : used for one component system
(ii) T-C Diagram : used for two component system
APPLICATIONS OF PHASE RULE TO ONE COMPONENT
SYSTEM The water system:
Water exists in three possible phases namely
solid, liquid and vapour. Hence there
can be
three forms of equilibria.
Solid < -- -- > Liquid
Liquid < -- -- > Vapour
Solid < -- -- > Vapour
Each of
the above equilibrium involves two phases. The phase diagram for the water
system is shown in the figure.
This
phase diagram contains curves, areas,
and triple.
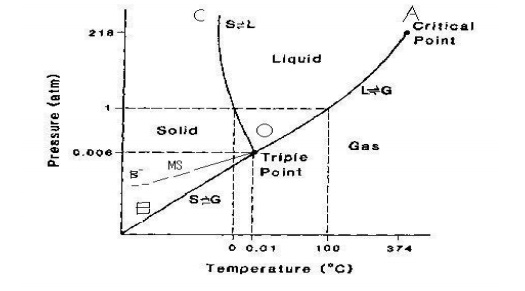
(i)Curve OA
The
curve OA is called vaporisation curve, it represents the equilibrium between
water and vapour. At any point on the curve the following equilibrium will
exist.
Water < -- -- > Water vapour
The degree of freedom of the system is one,
i.e, univariant.
This is predicted by the phase rule.
F=C-P+2;
F=1-2+2; F=1
This
equilibrium (i.e. Line OA) will extend up to the critical temperature (347o
C). Beyond the critical temperature the equilibrium will disappear only water
vapour will exist.
(ii) Curve OB
The
curve OB is called sublimation curve of ice, it represents the equilibrium
between ice and vapour. At any point on the curve the following equilibrium
will exist.
ICE <
-- -- > VAPOUR
The degree of freedom of the system is one, i.e. univariant. This is predicted by the
phase rule.
F = C –
P + 2; F = 1-2=2 ; F=1
This
equilibrium (line OB) will extend up to the absolute zero (-273o C),
where no vapour can be present and only ice will exist.
iii) Curve OC
The
curve OC is called melting point curve of ice, it represents the equilibrium
between the ice and water. At any point on the curve the following equilibrium
will exist.
Ice < -- -- > water
The
curve OC is slightly inclined towards pressure axis. This shows that melting
point of ice decreases with increase of pressure.
The
degree of freedom of the system is one i.e., univariant. iv) point O
(triple point)
The
three curves OA ,OB ,OC meet at a point “O” ,where three phases namely solid
,liquid and vapour are simultaneously at equilibrium .
This
point is called triple point, at this point the following equilibrium will
exist.
Ice < -- -- > water < -- -- > vapour
![]()
![]() The degree of freedom of the system is zero i.e., nonvariant.This is predicted by the
phase rule. F=C-P+2; F=1-3+2=0
The degree of freedom of the system is zero i.e., nonvariant.This is predicted by the
phase rule. F=C-P+2; F=1-3+2=0
Temperature
and pressure at the point “O” are 0.0075 oC and 4.58 mm
respectively.
(v) Curve OB’: Metastable equilibrium
The
curve OB’ is called vapour pressure curve of the super-cool water or metastable
equilibrium where the following equilibrium will exist.
Super-cool water < -- -- > vapour
Sometimes
water can be cooled below O oC without the formation of ice, this
water is called super –cooled water. Super cooled water is unstable and it can
be converted in to solid by seeding or by slight disturbance.
vi) Areas
Area
AOC, BOC, AOB represents water, ice and vapour respectively .The degree of the
freedom of the system is two.i.e. Bivariant.
This is
predicted by the phase rule
F=C-P=2; F=1-1+2; F=2
Two component alloy system or multi component
equilibria Reduced phase rule or condensed system
The system in which only the solid and liquid are considered and
the gas phase is ignored is called a condensed system.since pressure kept
constant, the phase rule becomes
F’ = C –
P + 1
This
equation is called reduced phase rule.
Classification of two component system
Based on
the solubility and reactive ablity, the two component systems are classified in
to three types.
1. Simple
eutectic formation - A
binary system consisting of two substances, which are completely miscible in the liquid state, but completely immiscible
in the solid state, is known as eutectic (easy melt) system. They do not react
chemically. Of the different mixtures of the two substances, the mixture having
the lowest melting point is known as the eutectic mixture.
2. a) formation of compound with congruent melting
point
b) Formation of compound with incongruent melting
point
3. Formation of solid solution
Thermal analysis or cooling curve
Thermal analysis is a method involving a study of the cooling
curves of various compositions of a system during solidification. The form of
the cooling curve indicates the composition of the solid.
Ex: 1.
Cooling curve of a pure solid. Ex: 2. Cooling curve of a mixture A + B.
A cooling curve is a line graph
that represents the change of phase of matter, typically from a
gas to a solid or a liquid to a solid.
The
independent variable (X-axis) is time and the dependent variable (Y-axis) is
temperature. Below is an example of a cooling curve.
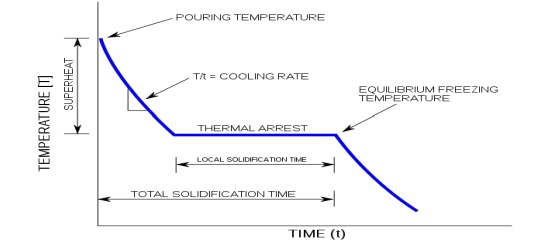
The
initial point of the graph is the starting temperature of the matter, here
noted as the "pouring temperature". When the phase change occurs
there is a "thermal arrest", that is the temperature stays constant.
This is because the matter has more internal energy as a liquid or gas than in the state that it is cooling to. The amount of
energy required for a phase change is known as latent heat. The
"cooling rate" is the slope of the cooling curve at any point.
A Pure substance in the fused or liquid state is allowed to cool
slowly. The temperature is noted at different times.when represented
graphically the rate of cooling will be a continuous from ‘a’ to ‘b’.
When the
freezing point is reached and solid making its appearance there will be a break
in the continuity of the cooling curve.The temperature will thereafter remain
constant until the liquid is completely solidified.Thereafter the fall in
temperature wil again become continuous.
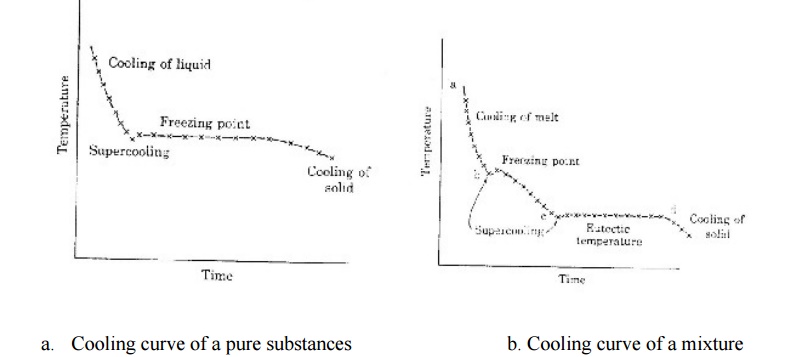
a. Cooling curve of a pure substances b. Cooling curve of a mixture
If a mixture of two solids in the fused state is cooled
slowly we get a cooling curve .
Here
also first a continuous coling curve will be obtained as long as the mixture is
in the liquid state .
When a
solid phase begins to form there will be a break in the cooling curve .But the
temperature will not remain constant unlike in the case of cooling of a
purified substance.The temperature will decrease continuously but at a
different rate.The fall of temperature will continue till the mixture forms a
eutectic and the eutectic point is reached.
The
temperature will thereafter remain constant until solidification is complete .
Thereafter the fall of temperature will become uniform ,but the rate of fall
will be different from that for a pure substance.
Uses of cooling curves
i)
Percentage
purity of the compounds can be noted from the cooling curve.
ii)
The
behaviour of the compounds can be clearly understood from the cooling curve.
iii)
The
procedure of thermal analysis can be used to derive the phase diagram of any
two
component system.
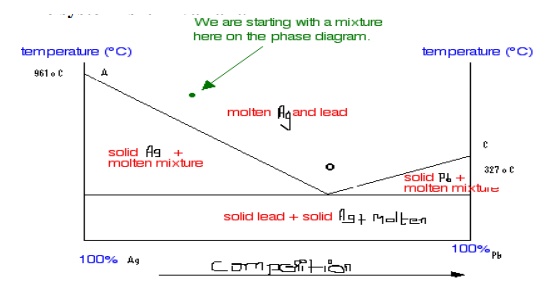
BINARY ALLOY SYSTEM OR THE SIMPLE EUTECTIC
SYSTEM The Lead – Sliver system
Since the system is studied at constant
pressure,the vapour phase is ignorned and the condensed phase rule is rule is
used.
F I= C-P+1
The phase diagram of lead –sliver system is
shown in the figure It contains lines,areas and the eutectic point.
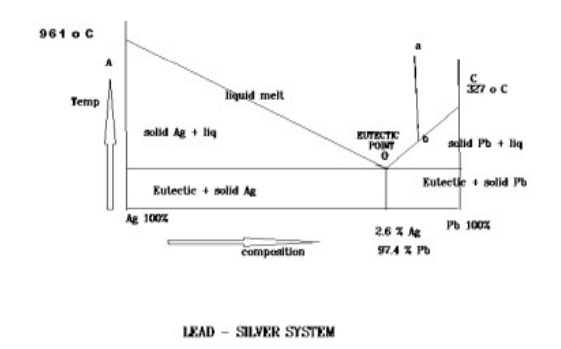
i) curve AO
The
curve AO is known as freezing point curve of sliver.
Along
the curve AO, solid Ag and the melt are in equilibrium.
Solid Ag
< -- -- > melt
According
to reduced phase rule
F’=C-P+1
C=2
P=2
F’=1
The
system is univariant
ii) curve BO
The
curve BO is known as freezing point curve of lead .
Along
the curve BO, solid Pb and the melt are in equilibrium.
Solid
Pb < -- -- > melt
According
to reduced phase rule
F’=C-P+1
C=2
P=2
F’=1
The
system is univariant.
iii) Point “ O ” (eutectic point)
The
curves AO and BO meet at point ‘ O ‘ at a temperature of 303 o C ,where the
three phases are in equilibrium.
Solid Pb
+ soild Ag < -- -- > melt
According
to reduced phase rule
F’=C-P+1
C=2
P=3
F’=1
The
system is non-variant.
The
point “ O “ is called eutectic point or eutectic temperature and is
corresponding composition,97.4 % Pb and 2.6 % Ag ,is called eutectic composition.below
this point the eutectic compound and the metal solidfy.
iv) Areas
The area
above the line AOB has a single phase( molten Pb + Ag ). According to reduced
phase rule
F’=C-P+1
C=2
P=1
F’=2
The
system is bi-variant.
The area
below the line AO ,OB and point “O” have two phases and hence the system is
univariant.
According
to reduced phase rule F’=C-P+1
C=2
P=2
F’=1
The
system is uni-variant.
The process of raising the relative proportion
of Ag in the alloy is known as pattinson’s process.
The Pattinson process was patented
in 1833. It depended on well-known material properties; essentially that lead
and silver melt at different temperatures. The equipment consisted of a row of
about 8-9 iron pots, which could be heated from below. Agentiferous lead was
charged to the central pot and melted. This was then allowed to cool, as the
lead solidified, it was skimmed off and moved to the next pot in one direction,
and the remaining metal was then transferred to the next pot in the opposite
direction. The process was repeated in the pots successively, and resulted in
lead accumulating in the pot at one end and silver in that at the other. The
process was economic for lead containing at least 250 grams of silver per ton.
Uses of
eutectic system
1.suitable
alloy composition can be predicted with the help of eutectic systems.
2.eutectic systems are used in preparing solders ,used for joining two metal
pieces together.
Melting point
It is
the temperature at which the solid and liquid phases, having the same
composition ,are in
equilibrium.
Solid
A < -- -- > solid B
Eutectic point
It is
the temperature at which two solids and a liquid phase are in equilibrium.
Solid A
+ solid B < -- ---> Liquid
Triple point
It is
the temperature at which three phases are in equilibrium.
Solid < -- ---> liquid
< -- ---> vapour
![]() By definition ,
By definition ,
All the
eutectic points are melting points, but all the melting points need not be
eutectic points. ll ly , all the eutectic points are triple points
,but all the triple points need not be eutectic points.
Uses (or) merits of phase rule
1. It is a convenient method of
classifying the equilibrium states in terms of phases ,components and degree of
freedom.
2. It
helps in deciding whether the given number of substances remain in equilibrium
or not.
Limitations of phase rule
1.phase
rule can be applied for the systems in equilibrium.
2.only
three variables like P,T & C are considered ,but not electrical, magnetic
and gravitational forces.
Definition
An alloy is defined as “homogeneous solid
solution of two or more different element one of which at least is essentially
a metal”. Alloy containing Hg as a constituent element are called amalgams.
Properties
of alloys
1)
Alloy
are harder less malleable and possess lower melting point than their component
metals
2)
Alloys
possess low electrical conductivity
3)
Alloys
resist corrosion and the action of acids
Importance
or need of making alloys
1.
To increase the hardness of the metal Example
Gold and silver are soft metal they are alloyed
with copper to make them hard
2. To
lower the melting points of the metal Example
Wood metal (an alloy of lead, bismuth, tin and
cadmium) melts at 60.5⁰c which is far below the melting points of any
of these constituent metals
3. To resist the corrosion of the metal
Example
Pure iron rested but when it is alloyed with
carbon chromium (stainless steel) which resists corrosion
4. To
modify chemical activity of the metal
Example
Sodium amalgam is less active than sodium but
aluminium amalgam is more active than aluminium
5. To
modify the colour of the metal Example
Brass an alloy of copper (red) and size
(silver-white) is white colour.
6. To
get good casting of metal Example
An alloy of lead with 5% tin and 2% antimony is
used fro casting printing type due toits good casting property
Functions
(or) effects of alloying elements
Addition of small amount of certain metals such
as Ni, Cr, Mo, Mn, Si, v and Al impart special properties like hardness,
tensile strength, resistance to corrosion and coefficient of expansion on
steel. Such products are known as special steel or alloy steels Some important
alloying element and their functions are given in table
CLASSIFICATION (OR) TYPES OF ALLOYS
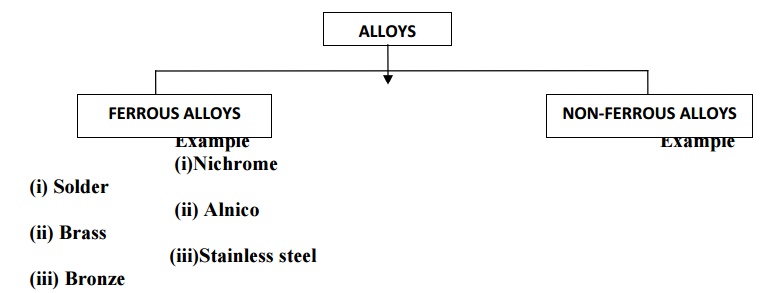
FERROUS ALLOYS OR ALLOY STEELS
Ferrous alloys are the type of steels in which
the elements like Al,B,Cr,Co,Cu,Mn are present in sufficient quantities, in
addition to carbon & iron.
PROPERTIES
1. High yield point & strength
2. Sufficient formability,ductility &
weldability
3. Corrosion & abrasion resistant
4. Less distortion & cracking
5. High temperature strength
IMPORTANT FERROUS ALLOYS (i)NICHROME
Nichrome is an alloy of nickel & chromium
COMPOSITION
Nickel –
60%
Chromium
– 12%
Iron –
26%
Manganese
– 2%
PROPERTIES
1. Good resistance to oxidation & heat
2. High melting point & electrical resistance
3. Withstand heat up to 1000-1100⁰C
USES
1. Used for making resistance coils,heting
elements in stoves & electric irons
2. Used in making parts of boilers,steam lines
stills,gas turbines,aero engine valves,retorts,annealing boxes.
(ii)ALNICO
Alnico is an alloy of aluminium-nickel-cobalt .
COMPOSITION
Aluminium
– 8-12%
Nickel –
14-28%
Cobalt –
5-35%
PROPERTIES
1. Excellent magnetic properties & high
melting point
2. Magnetized to produce strong magnetic fields as
high as 1500 gauss
TYPES OF
ALNICO ALLOYS
Alnico alloys are of two types
1. ISOTROPIC ALNICO
It is effectively magnetized in any direction
2.ANISOTROPIC
ALNICO
It possess preffered direction of magnetization.
Anisotropic alnico possesses greater magnetic
capacity in their preffered orientation than isotropic alnico.
USES
1. Used as permanent magnets in
motors,generators,radio speakers microphones,telephone receivers &
galvanometers.
(iii)STAINLESS
STEELS (or)CORROSIOPN RESISTANT STEELS
· These are alloy steels containing chromium together with other elements such
as nickel,molybdenum,etc.
· Chromium-16%
or more
· Carbon-0.3-1.5%
PROPERTIES
1. Resist corrosion by atmospheric gases &
also by other chemicals.
2.
Protection against corrosion is due to the
formation of dense, non-porous,tough film of chromium oxide at the metal
surface. If the film cracks, it gets automatically healed up by atmospheric
oxygen
TYPES OF STAINLESS STEEL
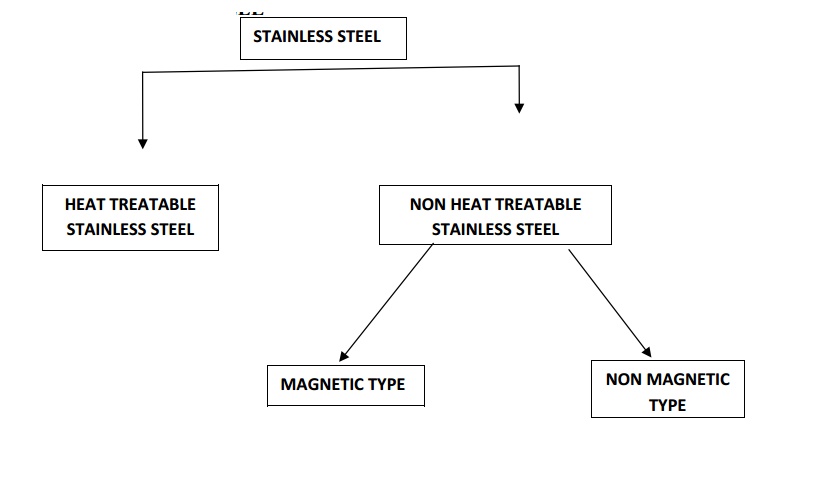
1. HEAT TREATABLE STAINLESS STEEL
COMPOSITION Carbon-1.2%
Chromium-less
than 12-16%
PROPERTIES
Magnetic,tough & can be worked in cold
condition
USES
1. Can be used up to 800⁰C
2. Good resistant towards weather & water
3. In making surgical
instruments,scissors,blades,etc.
2.HEAT
TREATABLE STAINLESS STEEL
PROPERTIES
· Possess less strength at high temperature
· Resistant to corrosion
TYPES OF NON HEAT TREATABLE STAINLESS STEEL
(a)MAGNETIC TYPE
COMPOSITION
Chromium-12-22%
Carbon-0.35%
PROPERTIES
1. Can be forged,rolled & machined
2. Resist corrosion
USES
Used in making chemical equipments&
automobile parts.
(b)NON MAGNETIC TYPE
COMPOSITION
Chromium-18-26%
Nickel-8-21%
Carbon-0.15%
Total % of Cr & Ni is more than 23%.
EXAMPLE:18/8 STAINLESS STEEL COMPOSITION: Chromium-18%
Nickel-8%
PROPERTIES
1. Resistance to corrosion.
2. Corrosion resistance is increased by adding
molybdenum
USES
In making household utensils,sinks,dental &
surgical instruments.
NON FERROUS ALLOYS
· Do not contain iron as one of the main
constituent.
· Main constituents are
copper,aluminium,lead,tin,etc.
PROPERTIES
1. Softness & good formability
2. Attractive (or) very good colours
3. Good electrical & magnetic properties
4. Low density & coefficient of friction
5. Corrosion resistance
IMPORTANT NON FERROUS ALLOYS 1. COPPER ALLOYS
(BRASS)
Brass contains mainly copper & zinc
PROPERTIES
· Greater strength, durability &
machinability
· Lower melting points than Cu & Zn
· Good corrosion resistance & water
resistance property
2.BRONZE(COPPER
ALLOY)
Bronze contains copper & tin
PROPERTIES
· Lower melting point
· Better heat & electrical conducting
property
· Non-oxidizing,corrosion resistance & water
resistance property.
3.SOLDERS
Solders are low- melting alloys of tin &
lead
PROPERTIES
Solder is melted to join metallic surfaces ,especially
in the fields of electronic &
plumbing
USES
1. Used in electrical industry
2. Alloy with 50% tin is general-purpose solder
3. For sealing automotive radiator cores.
4. As fuses for fire-extinguishing
equipments,boiler plugs,etc.
Heat treatment of alloys (steel)
Heat treatment is defined as”
the process of heating and cooling of solid steel article under carefully
controlled condition”. During heat treatment certain physical properties are
altered without altering its chemical composition
Objectives (or) purpose of heat treatment
Heat treatment causes
i.
Improvement
in magnetic and electrical properties
ii.
Refinement
of grain structure
iii.
Removal
of the imprisoned trapped gases
iv.
Removal
of internal stress
v.
Improves
fatique and corrosion resistance
Types of heat treatment of alloys (steel) 1.
Annealing
Annealing means softening.
This is done by heating the metal to high temperature followed by slow cooling
in a furnace.
Purpose
of annealing
i.
It
increases the machinability
ii.
It also
removes the imprisoned gases
Types of
annealing
Annealing can be done in two types
i.
Low
temperature annealing (or) process annealing
ii.
High
temperature annealing 9or) full annealing
Low
temperature annealing (or)process annealing
It involves in heating steel
to a temperature below the lower critical point followed by slow cooling
Purpose
1. It improves mashinability by reliving the
internal stress or internal strain
2. It increases ductility and shock resistance
3. It reduce hardness
(i)
High temperature annealing (or) fault annealing
It
involves in heating to a temperature about 30 to 50⁰C above the higher critical temperature and
holding it at that temperature for sufficient time to allow the internal
changes to take place and then cooled room
temperature
The approximate annealing temperature of various
grades of carbon steel
are
1. Mild steel=840-870⁰c
2. Medium carbon steel=780-840⁰c
3. High carbon steel=760-780⁰c
Purpose
1. It increases the ductility and machinability
2. It makes the steel softer, together with an
appreciable increases in its toughness
2.Hardening
(or) quenching
· It is the process of heating steel beyond the
critical temperature and then suddenly cooling it either in oil or brine water
or some other fluid.
· The faster the rate of cooling harder will be
the steel produced.
· Medium and high carbon steel can be hardened
but low carbon steel cannot hardened
Purpose
1. It increases its resistance to wear ability ,to
cut other metal and strength .
2. It increases abrasion resistance.
3. Used for making cutting tools.
3.
TEMPERING
· It is the process of heating the already
hardened steel to a temperature lower than its own hardening temperature &
then cooling it slowly.
· The reheating controls the development of the
final properties
· Thus,
(a)For retaining strength
& hardness, reheating temperature should not exceed 400⁰C.
(b) For developing better
ductility & toughness, reheating temperature should be within 400-600⁰C.
Purpose
1. It removes stress &strains that might have
developed during quenching.
2. Increased toughness & ductility.
3. Used for cutting tools like blade,cutters etc.
4. NORMALISING
It is the purpose of heating steel to a
definite temperature (above its higher
critical temperature) & allowing it to cool gradually in air. Purpose
1. Recovers homogeneity
2. Refines grains.
3. Removes internal stresses
4. Increases toughness
5. Used in engineering works
NOTE: The difference between normalised &
annealed steel are
1. A normaled steel will not be as soft as
annealed steel.
2. Also normalizing takes much lesser time than
annealing.
5.CARBURIZING
· The mild
steel article is taken in a cast iron box within containing small pieces of
charcoal(carbon material).
· It is
heated to about 900 to 950⁰C & allow it for sufficient time,so that
the carbon is absorbed to required depth .
· The
article is then allowed to cool slowly within the box itself.
· The
outer skin of the article is converted into high carbon steel containing
about 0.8 to 1.2% carbon. Purpose
To produce hard surface on steel article
6.NITRIDING
· Nitriding
is the process of heating the metal alloy in presence of ammonia to about 550⁰C.
· The
nitrogen (obtained by the dissociation of ammonia) combines with the surface of
the alloy to form hard nitride.
Purpose
To get super-hard surface.
Related Topics