Chapter: Medical Immunology: Immune System Modulators
Immunopotentiation
IMMUNOPOTENTIATION
Many compounds and biological substances have been used in attempts to restore normal immune system function in clinical conditions in which it is believed to be functionally altered. All of these types of therapeutic interventions fall under the general designation of immunopotentiation, which can be defined as any type of therapeutic intervention aimed at restoring the normal function of the immune system.
A. Biological Response Modifiers
These include a variety of soluble compounds that allow the various elements of the im-mune system to communicate with one another. This communication network allows for “upregulation” and coordination of immune responses when needed and “downregulation” of immune responses when no longer needed by the host.
1. Structure
All BRMs have a surprising degree of structural similarity to one another; this is felt to be reflective of an early gene reduplication event, which occurred as the immune system of mammals evolved.
2. Function
The BRMs appear to form a very delicate network of communication signals between the four principal mononuclear leukocyte subsets that participate in the immune response. We still do not completely understand how these interactions occur; however, the release of these signals appears to be different from hormones. Hormones tend to act at a site far dis-tant from the original cell that secreted the signal; in contrast, BRMs appear to work in the immediate vicinity of the cell type that secretes them. The timing of the release of BRMs coupled with the intensity of their secretion appears to provide the overall balance of sig-nals required to orchestrate the immune response.
3. Cellular Sources
The most significant sources of BRM compounds are lymphocytes, macrophages, and den-dritic cells. T lymphocytes produce a wide range of BRMs, including:
1. Interleukin-2 (IL-2), which induces the proliferation of T lymphocytes, B lymphocytes, and NK cells. IL-2 is also capable of upregulating the tumor-killing capabilities of CD8+ T lymphocytes and NK cells.
2. Interleukin-3 (IL-3), a growth factor for stem cells in the bone marrow that stimulates the production of many types of leukocytes from the bone marrow when needed.
3. Interleukin-4 (IL-4), the main determinant of TH 2 differentiation; it also stim-ulates B lymphocytes to proliferate and induces the synthesis of IgE antibodies.
4. Interleukin-5 (IL-5), which has chemotactic and activating properties for eosinophils.
5. Interleukin-10 (IL-10), which downregulates the synthesis of other interleukins and turns-off both TH1 and TH 2 activities.
6. Granulocyte-monocyte colony-simulating factor (GM-CSF), which stimulates the bone marrow to produce both granulocytes and monocytes. It also appears capable of upregulating the spontaneous killing capability of monocytes.
7. γ-Interferon (IFNγ ), the main mediator responsible for the activation of mono-cytes and macrophages, which is also released by activated NK cells.
Natural killer cells also secrete a series of BRMs, particularly α-interferon (IFN-α ), an antiviral agent that promotes the activation of NK lymphocytes in vitro. FN-α is also se-creted by monocytes, macrophages, and many other cell types, but its main source has been recently identified as being the lymphoid-derived dendritic cells (DC2). In addition to its antiviral and NK-activating properties, α-interferon appears capable of directly inhibiting the growth of certain types of tumor cells, such as the cells that proliferate in hairy cell leukemia , chronic myelold leukemia, and melanoma.
Mononuclear phagocytes (monocytes and macrophages) share with NK lymphocytes the ability to secrete α-interferon. In addition, they secrete several types of interleukins and cytokines. Some of them (IL-6, IL-8, and TNF) are also produced by T lymphocytes.
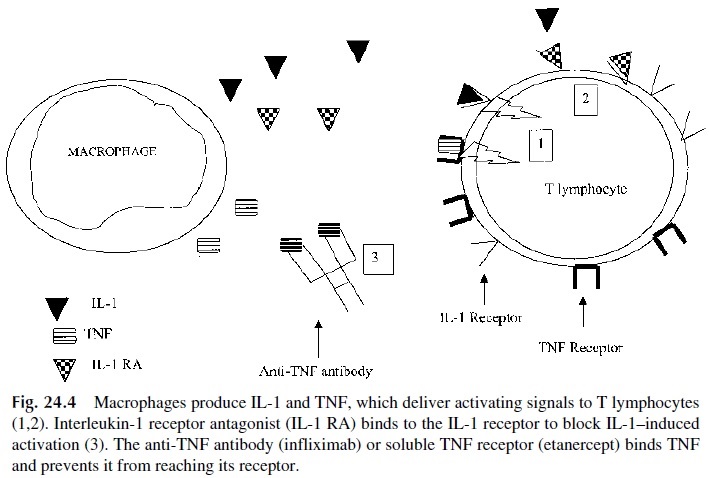
1. Interleukin-1 (IL-1), also produced by other antigen-presenting cells, including B lymphocytes, promotes the early phases of the B- and T-lymphocyte–activa-tion processes.
2. Interleukin-6 (IL-6), which promotes B-lymphocyte differentiation and im-munoglobulin secretion.
3. Interleukin-8 (IL-8), a chemotactic factor for T lymphocytes and neutrophils.
4. Interleukin-12 (IL-12), responsible for the activation and differentiation of TH1 lymphocytes.
5. Tumor necrosis factor (TNF) has a variety of effects, particularly causing the death of certain types of tumor cells.
6. Two types of hematopoietic factors, G-CSF and M-CSF. M-CSF promotes the production and activation of monocytes, and G-CSF stimulates the production of granulocytes from stem cells in the bone marrow.
4. Clinical Applications
Of the interleukins, interleukin-2 has been the most widely used. High-dose IL-2 induces a response in 15% of patients with disseminated melanoma and renal cell carcinoma. Despite the low response rate, the excitement regarding high-dose IL-2 centers on the duration of responses. Seven percent of these responses are complete and very durable, with median duration exceeding 40 months. Unfortunately, high-dose IL-2 is associated with severe side effects (e.g. hypotension, fluid retention, renal failure, and mental status changes), which necessitate administration in the hospital. Lower doses of IL-2 that can be administered safely on an outpatient basis are being extensively investigated in experimental immune re-constitution protocols in AIDS patients and to treat melanoma and renal cell carcinoma. Interleukin-12 has also been used in AIDS patients with the goal of en-hancing the differentiation of TH1 cells, although to a more limited extent.
Interferon-γ has been extensively studied as an inmunomodulator, particularly in pa-tients with chronic granulomatous disease .
Interferon-α induces antiviral, antitumor, and immunomodulatory effects. It acti-vates their target cells by first binding to specific interferon receptors on the cell surface. The binding of interferon-α induces a signaling cascade, the end result of which is to in-duce the synthesis of several effector proteins (e.g., 2’ ,5’ -oligo-A synthetase, Mx protein, and protein kinase). The upregulation of these effector proteins is important in its antiviral response but may also play a role in its antitumor response. The antitumor activity of in-terferon-α may also involve oncogene modulation (e.g., c-myc, c-fos) and induction of pro-teins (e.g., indolamine 2,3-dioxygenase) that inhibit macromolecule synthesis essential for tumor cell survival. Interferon-α also has indirect effects by activating macrophages and natural killer cells and upregulating expression of cell surface proteins (e.g., HLA class I, B7, and ICAM-1) on tumor cells.
Although the exact mechanism of action of interferon-α in oncology is not well de-fined (i.e., direct antiproliferative vs. immunomodulatory effect), this BRM is extensively used in the chronic phase of chronic myeloid leukemia, as adjuvant therapy after surgery in patients with a high risk of recurrence from melanoma, and for the treatment of AIDS-re-lated Kaposi’s sarcoma and follicular lymphomas. Interferon-α is also used clinically to treat chronic hepatitis B and C. Despite the numerous dosage regimens used in clinical practice, the universal side effects associated with interferon-α therapy are fatigue, flu-like symptoms (i.e., fever and chills, headache), and myalgias.
Interferon-β , like interferon-α , exhibits antiviral, antiproliferative, and immunomod-ulatory activity. Interferon-β is effective in reducing the severity and frequency of exacer-bations of multiple sclerosis.
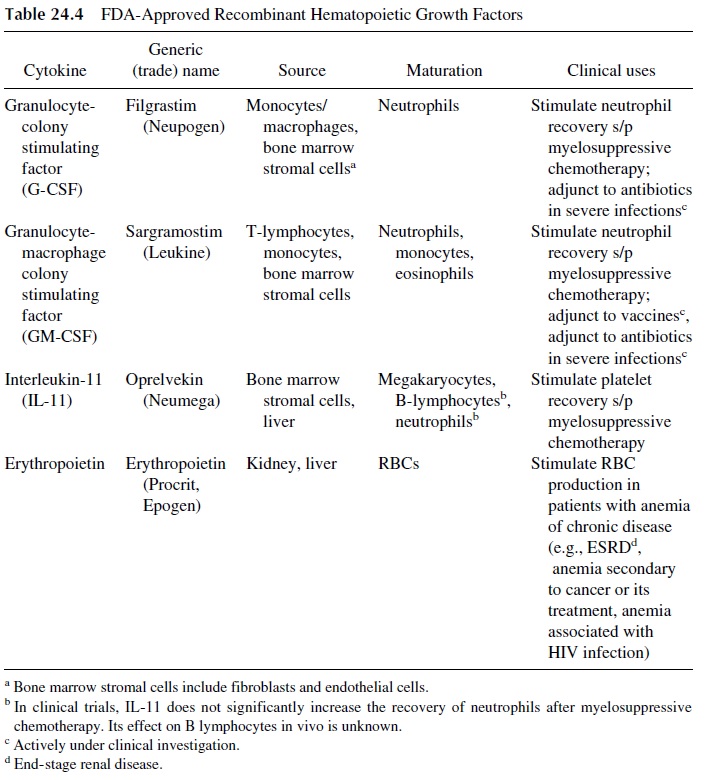
Randomized trials using the hematopoietic growth factors granulocyte colony-stim-ulating factor (G-CSF, filgrastim) or GM-CSF (sargramostim) postmyelosuppressive chemotherapy demonstrated their ability not only to accelerate neutrophil recovery but also to reduce the frequency and severity of infections, mainly bacterial, and to decrease antibi-otic use (Table 24.4). AIDS patients also develop neutropenia, due either to the viral in-fection itself (by unknown mechanisms) or as side effects of antiretroviral or other antimi-crobial drugs. The administration of G-CSF or GM-CSF to these patients may also be beneficial. The ability of G-CSF and GM-CSF to prevent infections is due to two effects: accelerated generation of neutrophils in the bone marrow and enhanced activity of neu-trophil function.
There is a strong correlation between the nadir of the neutrophil count and length of neutropenia from the chemotherapy with risk of bacterial infections. Typically, a neutrophil count lower than 500 cells/mm3puts a patient at increased risk of infection. However, the length of neutropenia also is critical. Patients with prolonged neutropenia status postchemotherapy (i.e.,
Both G-CSF and GM-CSF improve neutrophil function (phagocytosis, chemotaxis, and superoxide production). Based on this information, both G-CSF and GM-CSF are be-ing investigated as complementary therapy to antibiotics for the treatment of infectious dis-eases, such as severe foot ulcers in diabetics. In addition, human GM-CSF is the principal growth factor for the proliferation, maturation, and migration of dendritic cells and macrophages. Dendritic cells and macrophages play a critical role in antigen presentation for primary and secondary T-lymphocyte responses. Furthermore, GM-CSF increases the expression of MHC class II, CD80/86 co-stimulatory molecules and cell-adhesion molecules (e.g., ICAM-1) on antigen-presenting cells. Given this critical role of GM-CSF in the proliferation and maturation of dendritic cells and macrophages, the possible use of GM-CSF as a vaccine adjuvant has been investigated. Studies in laboratory animals demonstrated increased antibody production using GM-CSF as an adjuvant. Preliminary results of GM-CSF in conjunction with either the hepatitis B or influenza vaccine in hu-mans are encouraging, but further evaluation is necessary.
Two other hematopoietic growth factors available clinically are interleukin-11 and erythropoietin (Table 24.4). Platelets are fragments of megakaryocytes that, like all blood cells (e.g., RBCs, neutrophils), are derived from the pluripotent hematopoieitic stem cell. The commitment of a pluripotent hematopoietic stem cell toward a megakaryocyte is sub-ject to several influences, one of which is hematopoietic growth factors, namely interleukin-11, thrombopoietin, and interleukin-3. In patients receiving chemotherapy that causes clin-ically significant thrombocytopenia, recombinant interleukin-11 significantly shortened the period of thrombocytopenia (platelet count <50,000/ µL) and the number of platelet trans-fusions compared to placebo. It is important to note that most patients who receive myelo-suppressive chemotherapy get not only thrombocytopenia but also neutropenia. Interleukin-11 has minimal effect on neutrophils, and, therefore, it is not uncommon to give patients with severe myelosuppression secondary to chemotherapy both recombinant IL-11 and G-CSF. In addition, patients with anemia associated to myelosuppression (tumor-induced, virus-in-duced, or latrogenically induced) may benefit from erythropoietin. Erythropoietin stimu-lates the production of RBCs from the pluripotent hematopoietic stem cell.
Several BRMs have been used as primary or adjunct agents in the treatment of a va-riety of malignancies, with variable effects . There have also been many attempts to use BRMs to boost cellular immunity in immunodeficient patients, often with negative or conflicting results.
B. Intravenous Immunoglobulins
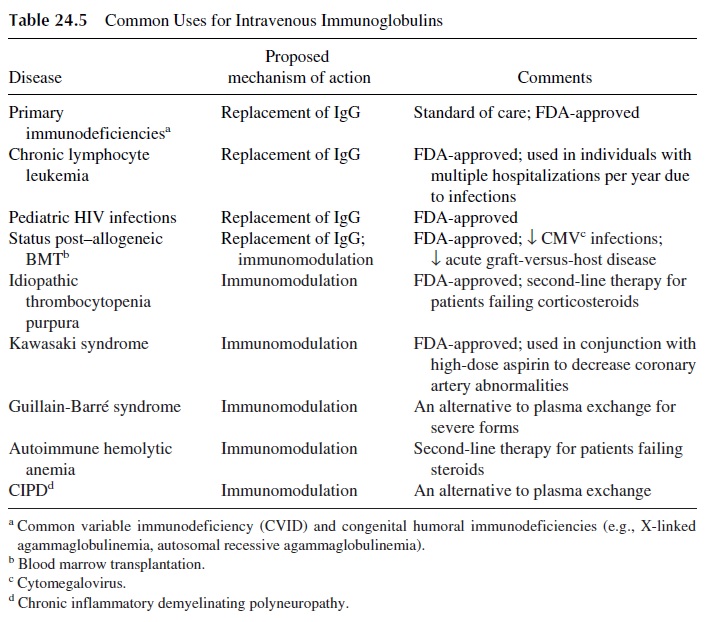
The administration of gammaglobulin as a way to transfer passive immunity to immunod-eficient patients is the oldest type of immunotherapy. Earlier preparations were administered intramuscularly, but later, to circumvent the limitations (e.g., painful injections, in-consistent absorption) of intramuscular immunoglobulin (IMIg), intravenous im-munoglobulin (IVIg) was introduced into clinical practice in the early 1980s. IVIg consists of concentrated polyclonal immunoglobulins, >90% IgG, prepared from pooled plasma collected from donors. In patients with a primary (e.g., common variable immunodefi-ciency) or secondary humoral immune deficiency (e.g., chronic lymphocytic leukemia), IVIg provides restoration of circulating IgG concentrations. The clinical benefit of restor-ing IgG concentrations with IVIg is decreased infections and hospitalization of these pa-tients.
Besides providing passive immunity, IVIg can also modulate the immune response. IVIg modulates the immune response by multiple mechanisms. Common uses of IVIg and the proposed mechanism of actions of IVIg are outlined in Table 24.5.
In many autoimmune disorders, an antibody directed against normal tissues (i.e., au-toantibody) binds to tissue and activates complement and antibody-dependent cellular cy-totoxicity (ADCC). For example, in idiopathic thrombocytopenia purpura (ITP), an au-toantibody against platelets causes immune destruction of the platelets. IVIg can modulate the autoantibody response by (1) saturating Fc receptors on phagocytes (e.g., neutrophils, macrophages) and preventing the engulfment of the autoantibody-coated platelets and by (2) providing the patient with an anti-idiotype antibody against the autoantibody. The anti-idiotype antibody binds the autoantibody in the variable region and prevents it from bind-ing to its antigen—in this example, platelets (Fig. 24.5). Also, the anti-idiotype antibodies
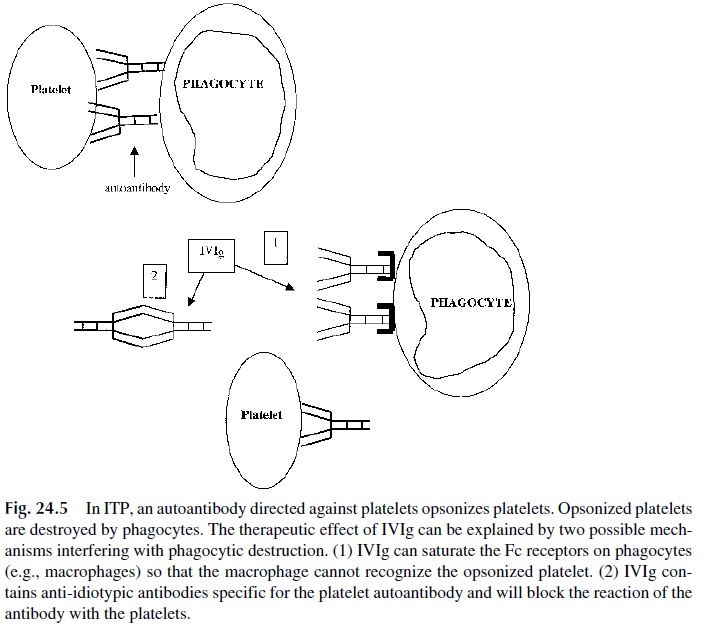
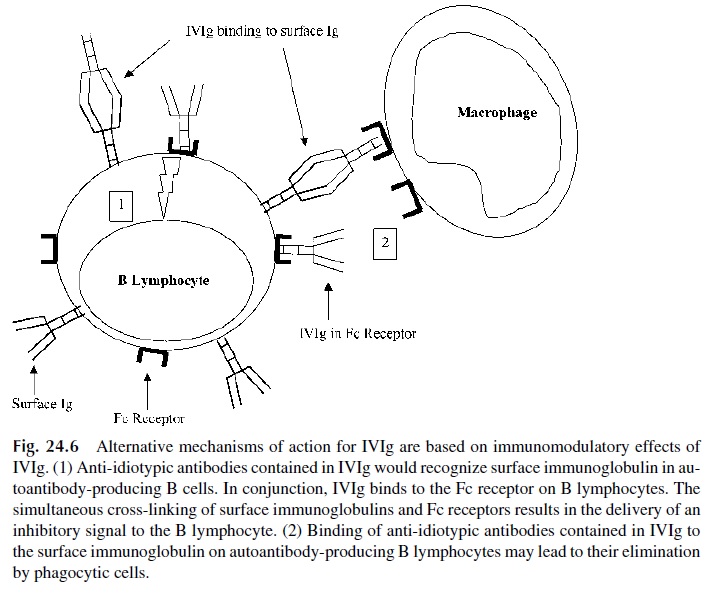
The binding of anti-idiotype to the surface immunoglobulin in combination with the binding of IVIg to the Fc receptor on B lymphocyte may induce apoptosis of the B lym-phocyte and block autoantibody production (Fig. 24.6). Recently, IVIg has been shown to contain antibodies that bind to and block the Fas receptor, thus interrupting the delivery of apoptotic signals to cells with upregulated Fas. IVIg has been shown to inhibit Fas-medi-ated cell death both in vitro and in vivo. The clinical applications of this property have yet to be defined.
C. Active Immunization as an Immunomodulating Intervention
Active immunization can be used for prevention of infectious diseases, , or to enhance the immunological defenses in patients already infected. The immunotherapeutic use of vaccines has been the object of experimental protocols in two areas:
1. HIV infection. The administration of low doses of killed HIV vaccine is cur-rently being tried in HIV-positive individuals, with the goal of stimulating the activity of TH1 helper lymphocytes, believed to be essential for the differentia-tion of cytotoxic T lymphocytes with antiviral activity .
2. Cancer. Anticancer vaccines have been the object of considerable interest andare also the object of ongoing trials .
3. Vaccines to stimulate resistance against antibiotic-resistant bacteria are also under devel-opment.
D. Dialyzable Leukocyte Extracts and Transfer Factor
In a series of classical experiments, Lawrence showed that the injection of an extract of lymphocytes from a tuberculin-positive donor to a tuberculin-negative recipient resulted in acquisition of tuberculin reactivity by the latter. Lawrence coined the term transfer factor to designate the unknown agent responsible for transfer of tuberculin sensitivity. Transfer factor has been used episodically to treat a variety of conditions, and the best results appear to have been obtained in the treatment of chronic mucocutaneous candidiasis, a rare form of cell-mediated immunodeficiency . Because no properly controlled tri-als have ever been performed with transfer factor, and because its chemical nature remains unknown, its place in immunotherapy remains marginal.
E. Thymic Hormones
Immunotherapeutic applications of thymic hormones have received increasing attention in recent years. Many peptides with thymic hormone–like activity have been isolated and described, including thymosin, facteur thymique serique (FTS, serum thymic factor), and thy-mopoietin. Although better characterized than transfer factor, the successful therapeutic ap-plications of thymic hormones remain the domain of anecdotal reports. For that reason, thymic hormones have never been recognized as clinically useful.
F. Bacterial and Chemical Immunomodulators
A variety of killed bacteria, several substances of bacterial origin, and chemical compounds have been used with the goal of activating the immune system in a variety of clinical con-ditions in which such activation should be beneficial.
1. Bacterial Immunomodulators
Bacille Calmette-Guérin (BCG) and Corynebacterium parvum have been extensively used for their adjuvant therapy in therapeutic protocols aimed at stimulating antitumoral im-munological mechanisms . At the cellular level, these bacteria appear mainly to activate macrophages.
2. Chemical Immunomodulators
Levamisole is an antihelminthic drug used in veterinary medicine that has been found to have immunostimulant properties. In some animal diseases it causes an apparent increase in host resistance to tumor cells. It acts on the cellular arm of the immune system and can restore impaired cell-mediated immune responses to normal levels but fails to hyperstimu-late the normal functioning immune system. Thus, it shows true immunomodulatory activ-ity.
In humans, levamisole has been reported to restore delayed hypersensitivity reac-tions in anergic cancer patients and to be of some benefit in the treatment of aphthous stomatitis, rheumatoid arthritis, systemic lupus erythematosus, viral diseases, and chronic staphylococcal infections. In patients with resected colorectal carcinomas, the adminis-tration of levamisole in conjunction with 5-fluorouracil decreased the frequency of re-lapses.
Isoprinosine (Inosine Prabonex, ISO) is a synthetic immunomodulatory drug recently approved for clinical use in the United States. It appears to be effective in a wide variety of viral diseases. This is probably due to the fact that this compound has both antiviral and im-munostimulating properties. As far as its effects on the immune system, isoprinosine po-tentiates cell-mediated immune responsiveness in vivo, and a major factor in its effective-ness against viral infections appears to be its ability to prevent the depression of cell-mediated immunity that has been shown to occur during viral infection and to persist for four to six weeks thereafter.
The clinical efficacy of ISO has been well documented in double-blind trials. Its ad-ministration results in striking decreases in both the duration of infection and severity of symptoms in a whole host of viral diseases, including viral influenza, rhinovirus infections, herpes simplex infections, herpes zoster, viral hepatitis, rubella, and viral otitis. Of partic-ular interest are the results of ISO therapy in subacute sclerosing panencephalitis (SSPE), a progressive disease due to a chronic measles virus infection, which results in complete debilitation and eventual death of the patient. ISO has been reported to halt the progression of SSPE in 80% of the patients when given in stages I and II of the disease, provided it is administered for at least 6 months. Indeed, ISO is the only agent to date with documented beneficial effects in SSPE patients.
Related Topics