Chapter: Mechanical : Advanced IC Engines : Alternate Fuels
Hydrogen as alternate fuels
Hydrogen as alternate fuels
Hydrogen
is a long-term renewable and less-polluting fuel. In addition hydrogen is clean
burning characteristics and better performance drives more interest in hydrogen
fuel. When it is burnt in an internal combustion engine, the primary combustion
product is water with no CO2. Although NOx emissions are formed when hydrogen
is used.
Combustive Properties of Hydrogen
Wide range of flammability
Compared
to nearly all other fuels, hydrogen has a wide flammability range (4-75% versus
1.4-7.6% volume in air for gasoline). This first leads to obvious concerns over
the safe handling of hydrogen. But, it also implies that a wide range of
fuel-air mixtures, including a lean mix of fuel to air, or, in other words, a
fuel-air mix in which the amount of fuel is less than the stoichiometric, or
chemically ideal, amount.
Small quenching distance
Hydrogen
has a small quenching distance (0.6 mm for hydrogen versus 2.0 mm for
gasoline), which refers to the distance from the internal cylinder wall where
the combustion flame extinguishes. This implies that it is more difficult to
quench a hydrogen flame than the flame of most other fuels, which can increase
backfire since the flame from a hydrogen-air mixture more readily passes a
nearly closed intake valve, than a hydrocarbon-air flame.
Flame velocity and adiabatic flame
Hydrogen
burns with a high flame speed, allowing for hydrogen engines to more closely
approach the thermodynamically ideal engine cycle (most efficient fuel power
ratio) when the stoichiometric fuel mix is used. However, when the engine is
running lean to improve fuel economy, flame speed slows significantly.
Flame
velocity and adiabatic flame temperature are important properties for engine
operation and control, in particular thermal efficiency, combustion stability
and emissions.
Minimum ignition source energy
The
minimum ignition source energy is the minimum energy required to ignite a
fuel-air mix by an ignition source such as a spark discharge. For a hydrogen
and air mix it is about an order of magnitude lower than that of a petrol-air
mix 0.02 mJ as compared to 0.24 mJ for petrol - and is approximately constant
over the range of flammability.
The low
minimum ignition energy of the hydrogen-air mix means that a much lower energy
spark is required for spark ignition. This means that combustion can be initiated
with a simple glow plug or resistance hot-wire. It also ensures prompt ignition
of the charge in the combustion chamber.
High diffusivity
Hydrogen
has very high diffusivity. This ability to disperse into air is considerably
greater than gasoline and is advantageous for two main reasons. Firstly, it
facilitates the formation of a uniform mixture of fuel and air. Secondly, if a
hydrogen leak develops, the hydrogen disperses rapidly. Thus, unsafe conditions
can either be avoided or minimized
Low density
The most
important implication of hydrogen’s low density is that without significant
compression or conversion of hydrogen to a liquid, a very large volume may be
necessary to store enough hydrogen to provide an adequate driving range. Low
density also implies that the fuel-air mixture has low energy density, which
tends to reduce the power output of the engine. Thus when a hydrogen engine is
run lean, issues with inadequate power may arise.
High auto-ignition temperature
The auto
ignition temperature is the minimum temperature required to initiate
self-sustained combustion in a combustible fuel mixture in the absence of an
external ignition. For hydrogen, the auto ignition temperature is relatively
high 585ºC. This makes it difficult of ignite a hydrogen–air mixture on the
basis of heat alone without some additional ignition source. This temperature
has important implications when a hydrogen–air mixture is compressed. In fact,
the auto ignition temperature is an important factor in determining what
maximum compression ratio an engine can use, since the temperature rise during
compression is related to the compression ratio.
The
temperature may not exceed hydrogen’s auto ignition temperature without causing
premature ignition. Thus, the absolute final temperature limits the compression
ratio. The high auto ignition temperature of hydrogen allows larger compression
ratios to be used in a hydrogen engine than in a hydrocarbon engine.
Advantages
of hydrogen as alternate fuel:
1. Hydrogen produces only water
after combustion.
2H2
+ O2 = 2H2O
2. When
hydrogen is burned, hydrogen combustion does not produce toxic products such as
hydrocarbons, carbon monoxide, and oxide of sulfur, organic acids or carbon
dioxides
3. Hydrogen
has some peculiar features compared to hydrocarbon fuels, the most significant
being the absence of carbon.
4. The
burning velocity is so high that very rapid combustion can be achieved.
5. The
density of hydrogen is 0.0838 kg/m3, which is lighter than air that it can
disperse into the atmosphere easily.
6. Hydrogen
has the highest energy to weight ratio of all fuels.
Disadvantages
of hydrogen as alternate fuels:
1. NOx
is formed as emission.
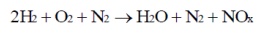
2. Storage
of hydrogen is more difficult as it leads to crack.
3. It is not
possible to achieve ignition of hydrogen by compression alone. Some
sources
of ignition have to be created inside the combustion chamber to ensure
ignition.
Formation
of NOx depends on the factors like
·
The air/fuel ratio
· The
engine compression ratio
·
The engine speed
·
The ignition timing
·
Whether thermal dilution is utilized
Related Topics