Chapter: Clinical Anesthesiology: Anesthetic Management: Hepatic Physiology & Anesthesia
Hepatic Physiology and Functional Anatomy
FUNCTIONAL ANATOMY
The liver is the heaviest organ in the body,
weighing approximately 1500 g in adults. It is separated by the falciform ligament into right and left
anatomic lobes;the larger right lobe has two additional smaller lobes at its
posterior–inferior surface, the caudate and quadrate lobes. In contrast,
surgical anatomy divides the liver based on its blood supply. Thus, the right
and left surgical lobes are defined by the point of bifurcation of the hepatic
artery and portal vein (porta hepatis);
the falciform ligament therefore divides the left surgical lobe into medial and
lateral segments. Surgical anatomy defines a total of eight segments.
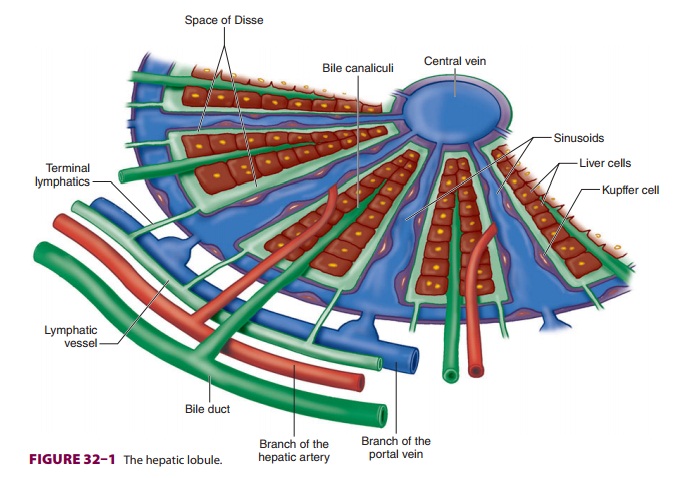
The liver is made up of 50,000–100,000
discrete anatomic units called lobules.
Each lobule is com-posed of plates of hepatocytes arranged cylindrically around
a centrilobular vein (Figure
32–1). Four to
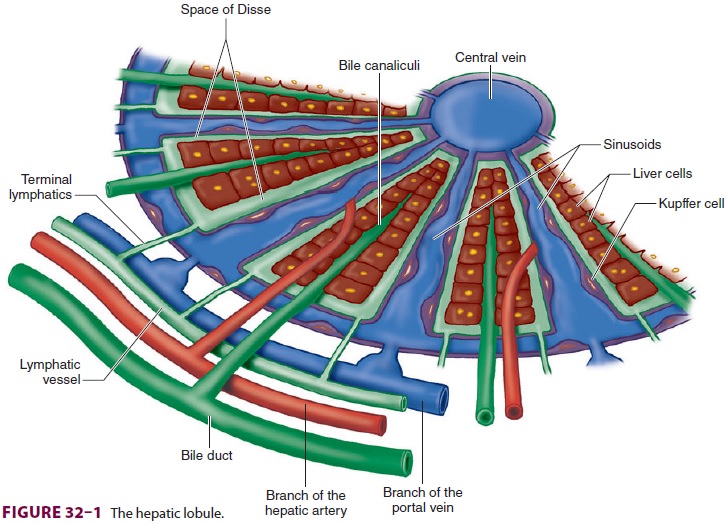
five portal tracts, composed of hepatic
arterioles, portal venules, bile canaliculi, lymphatics, and nerves, surround
each lobule.
In contrast to a lobule, an acinus,
the functional unit of the liver, is defined by a portal tract in the middle
and centrilobular veins at the periphery. Cells closest to the portal tract
(zone 1) are well oxygenated; those closest to centrilobular veins (zone 3)
receive the least oxygen and are most susceptible to injury.
Blood from hepatic arterioles and portal
venules comingle in the sinusoidal channels, which lie between the cellular
plates and serve as capillar-ies. These channels are lined by endothelial cells
and by macrophages known as Kupffer cells.
The Kupffer cells remove bacteria endotoxins, viruses, proteins and particulate
matter from the blood. The spaceof Disse lies
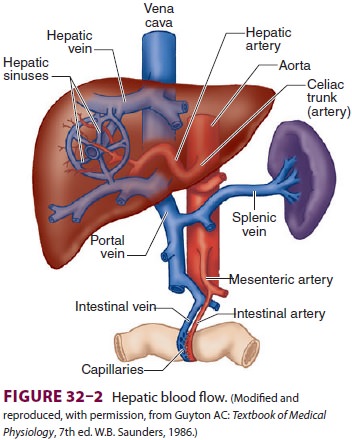
between the sinusoidal capillaries andthe hepatocytes. Venous drainage from the
central veins of hepatic lobules coalesces to form the hepatic veins (right,
middle, and left), which empty into the inferior vena cava (Figure
32–2). The caudate lobe is usually drained by its
own set of veins.
Bile canaliculi originate between hepatocytes within each plate and join
to form bile ducts. An extensive system of lymphatic channels also forms within
the plates and is in direct communication with the space of Disse.
The liver is supplied by sympathetic nerve fibers (T6–T11),
parasympathetic fibers (right and left vagus), and fibers from the right phrenic
nerve. Some autonomic fibers synapse first in the celiac plexus, whereas others
reach the liver directly via splanchnic nerves and vagal branches before
form-ing the hepatic plexus. The majority of sensory affer-ent fibers travel
with sympathetic fibers.
Hepatic Blood Flow
Normal hepatic blood flow is 25% to 30% of the car-diac output and is provided by the hepatic artery andportal vein. The hepatic artery supplies about 45% to 50% of the liver’s oxygen requirements, and the portal vein supplies the remaining 50% to 55% (Figure 32–2). Hepatic arterial flow seems to be dependent on metabolic demand (autoregulation), whereas flow through the portal vein is dependent on blood flow to the gastrointestinal tract and the spleen. A reciprocal, though somewhat limited, mechanism exists, such that a decrease in either hepatic arterial or portal venous flow results in a compensatory increase in the other.
The hepatic artery has α1-adrenergic
vasocon-striction receptors as well as β2-adrenergic, dopami-nergic (D1), and cholinergic vasodilator
receptors. The portal vein has only α1-adrenergic
and dopa-minergic (D1) receptors. Sympathetic
activation results in vasoconstriction of the hepatic artery and mesenteric
vessels, decreasing hepatic blood flow. β-Adrenergic stimulation vasodilates the hepaticartery; β-blockers reduce blood flow, and,
therefore, decrease portal pressure.
Reservoir Function
Portal vein pressure is normally only about
7–10 mm Hg, but the low resistance of the hepatic sinusoids allows relatively
large blood flows through the por-tal vein. Small changes in hepatic venous
tone and hepatic venous pressure thus can result in large changes in hepatic
blood volume, allowing the liver to act as a blood reservoir (Figure
32–3). A decrease in hepatic venous pressure, as
occurs during hemor-rhage, shifts blood from hepatic veins and sinusoids into
the central venous circulation and augments
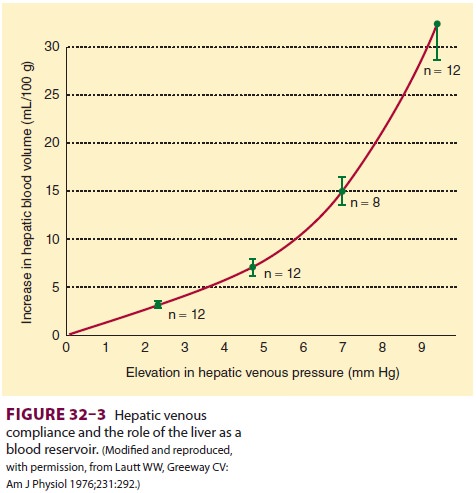
circulating blood volume. Blood loss can be reduced during liver surgery
by lowering the central venous pressure, thereby reducing hepatic venous
pressure and hepatic blood volume. In patients with conges-tive heart failure,
the increase in central venous pres-sure is transmitted to the hepatic veins
and causes congestion of the liver that can adversely affect liver function.
Metabolic Function
The abundance of enzymatic pathways in the
liver allows it to play a key role in the metabolism of car-bohydrates, fats,
proteins, and other substances (see Figure 32–4 and Table 32–1). The final products
ofcarbohydrate digestion are glucose, fructose, and galactose. With the
exception of the large amount of fructose that is converted by the liver to
lactate,
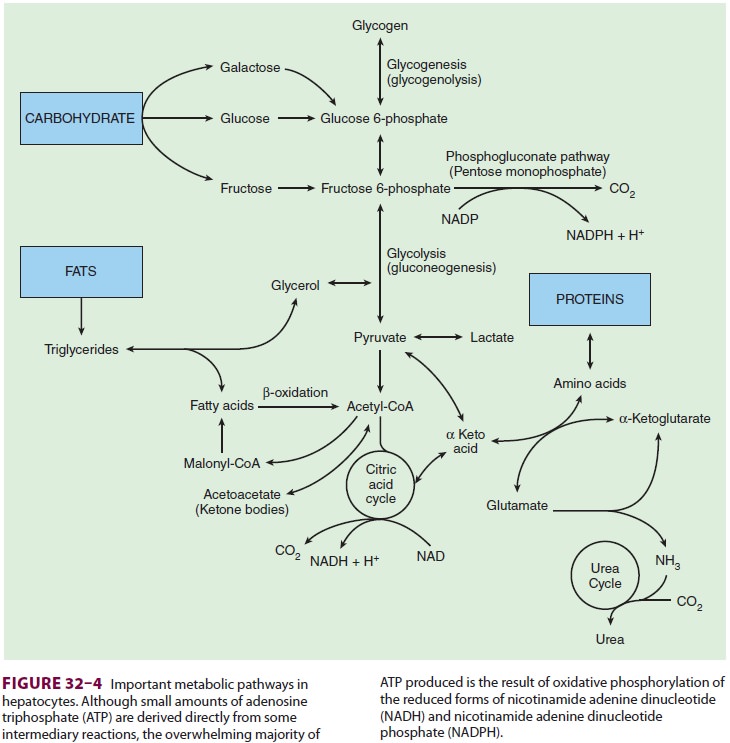
hepatic conversion of fructose and galactose into glucose makes glucose
metabolism the final com-mon pathway for most carbohydrates.
All cells utilize glucose to produce energy
in the form of adenosine triphosphate (ATP), either aero-bically via the citric
acid cycle or anaerobically via glycolysis. The liver and adipose tissue can
also uti-lize the phosphogluconate pathway, which provides energy and fatty
acid synthesis. Most of the glucose absorbed following a meal is normally
stored as gly-cogen, which only the liver and muscle are able to store in
significant amounts. When glycogen stor-age capacity is exceeded, excess
glucose is converted into fat. Insulin enhances glycogen synthesis, and
epinephrine and glucagon enhance glycogenolysis. Because glucose consumption
averages 150 g/day, and hepatic glycogen stores are normally only about 70
g/day, glycogen stores are depleted after 24 hr of fasting. After this period
of fasting, gluconeogenesis, the de novo synthesis of glucose, is
necessary to pro-vide an uninterrupted supply of glucose for other organs.
The liver and kidney are unique in their
capac-ity to form glucose from lactate, pyruvate, amino acids (mainly alanine),
and glycerol (derived from fat metabolism). Hepatic gluconeogenesis is vital in
the maintenance of a normal blood glucose concen-tration. Glucocorticoids,
catecholamines, glucagon, and thyroid hormone greatly enhance gluconeogen-esis,
whereas insulin inhibits it.
When carbohydrate stores are saturated, the liver converts the excess
ingested carbohydrates and proteins into fat. The fatty acids thus formed can
be used immediately for fuel or stored in adipose tis-sue or the liver for
later consumption. Nearly all cells utilize fatty acids derived from ingested
fats or synthesized from intermediary metabolites of car-bohydrates and protein
as an energy source—only red blood cells and the renal medulla are limited to
glucose utilization. Neurons normally utilize only glucose, but, after a few
days of starvation, they can switch to ketone bodies, the breakdown products of
fatty acids that have been synthesized by the liver as an energy source.
To oxidize fatty acids, they are converted into acetylcoenzyme A
(acetyl-CoA), which is then oxi-dized via the citric acid cycle to produce ATP.
The liver is capable of high rates of fatty acid oxidation and can form
acetoacetic acid (one of the ketone bodies) from excess acetyl-CoA. The
acetoacetate released by hepatocytes serves as an alternative energy source for
other cell types by reconversion into acetyl-CoA. Insulin inhibits hepatic
ketone body production. Acetyl-CoA is also used by the liver for the production
of cholesterol and phos-pholipids, which is necessary in the synthesis of
cellular membranes throughout the body.
The liver performs a critical role in protein metabolism. Without this
function, death usu-ally occurs within several days. The steps involved in
protein metabolism include: (1) deamination of amino acids, (2) formation of
urea (to eliminate the ammonia produced from deamination), (3)
inter-conversions between nonessential amino acids, and
formation of plasma proteins.
Deamination is necessary for the conversion of excess amino acids into
carbohydrates and fats. The enzymatic pro-cesses, most commonly transamination,
convert amino acids into their respective keto acids and pro-duce ammonia as a
byproduct.
Ammonia formed from deamination (as well as that produced by colonic
bacteria and absorbed through the gut) is highly toxic to tissues. Through a
series of enzymatic steps, the liver combines two molecules of ammonia with CO 2 to form
urea. The urea thus formed readily diffuses out of the liver and can then be
excreted by the kidneys.
Nearly all plasma proteins, with the notable
exception of immunoglobulins, are formed by the liver. These include albumin, α1-antitrypsin
and other proteases/elastases, and the coagulation fac-tors. Albumin is
responsible for maintaining a nor-mal plasma oncotic pressure and is the
principal binding and transport protein for fatty acids and a large number of
hormones and drugs. Consequently, changes in albumin concentration can
affect the concentration of the
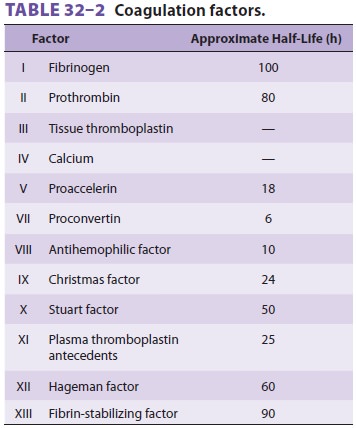
pharmacologically active, unbound fraction of many drugs.All coagulation
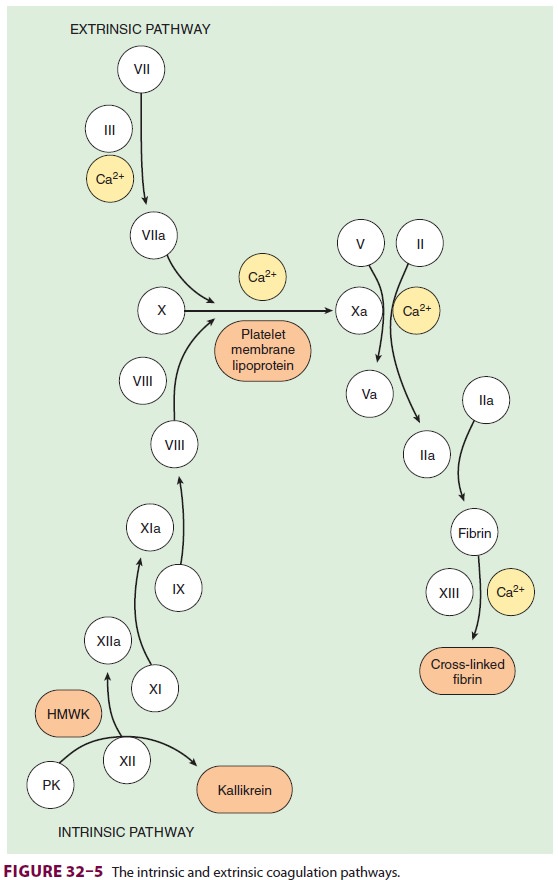
factors, with the exception of factor VIII and von Willebrand factor,
areproduced by the liver (see Table 32–2, Figure 32–5). Vascular endothelial
cells synthe-size factor VIII, levels of which are therefore usu-ally
maintained in chronic liver disease. Vitamin K is a necessary cofactor in the
synthesis of pro-thrombin (factor II) and factors VII, IX, and X. The
liver also produces plasma cholinesterase (pseudo-cholinesterase), an
enzyme that hydrolyzes esters, including some local anesthetics and some muscle
relaxants. Other important proteins formed by the liver include protease
inhibitors (antithrombin III, α2-antiplasmin,
and α1-antitrypsin), transport
pro-teins (transferrin, haptoglobin, and ceruloplasmin), complement, α1-acid glycoprotein, C-reactive
pro-tein, and serum amyloid A.
Drug Metabolism
Many exogenous substances, including most drugs, undergo hepatic
biotransformation, and the end-products of these reactions are usually either
inactivated or converted to more water-soluble substances that can be readily
excreted in bile or urine. Hepatic biotransformations are often cat-egorized as
one of two types of reactions. Phase
Ireactions modify reactive chemical groups throughmixed-function oxidases
or the cytochrome P-450 enzyme systems, resulting in oxidation, reduc-tion,
deamination, sulfoxidation, dealkylation, or methylation. Barbiturates and
benzodiazepines are inactivated by phase I reactions. Phase II reactions, which may or may not follow a phase I reaction,
involve conjugation of the substance with glucuro-nide, sulfate, taurine, or
glycine. The conjugated compound can then be readily eliminated in urine or
bile.
Some enzyme systems, such as those of cyto-chrome P-450, can be induced
by a few drugs, such as ethanol, barbiturates, ketamine, and perhaps
ben-zodiazepines. This can result in increased tolerance to the drugs’ effects.
Conversely, some agents, such as cimetidine and chloramphenicol, can prolong
the effects of other drugs by inhibiting these enzymes. Some drugs, including
lidocaine, morphine, vera-pamil, labetalol, and propranolol, have very high
rates of hepatic extraction from the circulation, and their metabolism is
therefore highly dependent upon the rate of hepatic blood flow. As a result, a
decrease in their metabolic clearance usually reflects decreased hepatic blood
flow rather than hepatocel-lular dysfunction.
The liver plays a major role in hormone, vitamin, and mineral metabolism. It is an important site for the conversion of thyroxine (T4) into the more active tri-iodothyronine (T3), and degradation of thyroid hor-mone is principally hepatic. The liver is also the major site of degradation for insulin, steroid hormones (estrogen, aldosterone, and cortisol), glucagon, and antidiuretic hormone. Hepatocytes are the principal storage sites for vitamins A, B12, E, D, and K. Lastly, hepatic production of transferrin and haptoglobin is important because these proteins are involved in iron hemostasis, whereas ceruloplasmin is important in copper regulation.
Bile Formation
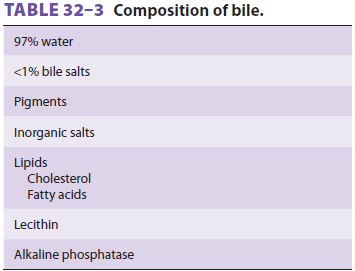
Bile (Table 32–3) plays an
important role in absorption of fat and excretion of bilirubin, cho-lesterol,
and many drugs. Hepatocytes continu-ously secrete bile salts, cholesterol,
phospholipids, conjugated bilirubin, and other substances into bile canaliculi.
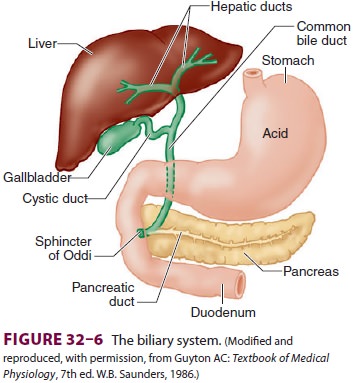
Bile ducts from hepatic lobules join and
eventu-ally form the right and left hepatic ducts. These ducts, in turn,
combine to form the hepatic duct, which together with the cystic duct from the
gallbladder becomes the common bile duct (Figure 32–6). The gallbladder serves as a reservoir for bile. The bile acids formed
by hepatocytes from cholesterol are essential for emulsifying the insoluble
components of bile and facilitating the intestinal absorption of lipids.
Defects in the formation or secretion of bile salts interfere with the
absorption of fats and fat-sol-uble vitamins (A, D, E, and K). Because of
normally
limited
stores of vitamin K, a deficiency can develop in a few days. Vitamin K deficiency is manifestedas a coagulopathy due to impaired
formation of prothrombin and of factors VII, IX, and X.
Bilirubin is primarily the end-product of
hemo-globin metabolism. It is formed from degradation of the heme ring in
Kupffer cells. Bilirubin is then released into blood, where it readily binds to
albu-min. Hepatic uptake of bilirubin from the circula-tion is passive, but
binding to intracellular proteins traps the bilirubin inside hepatocytes.
Bilirubin is conjugated by the hepatocytes, primarily with gluc-uronide, and
actively excreted into bile canaliculi.
Related Topics