Chapter: Pharmaceutical Drug Analysis: Flame Spectroscopy
Flame Spectroscopy
FLAME SPECTROSCOPY
INTRODUCTION
Metallic salts (or metallic compounds) after dissolution
in appropriate solvents when introduced into a flame (for instance : acetylene
burning in oxygen at 3200°C), turns into its vapours that essentially contain
mostly the atoms of the metal. Quite a few such gaseous metal atoms are usually
raised to a particular high energy level that enables them to allow the
emission of radiation characteristics features of the metal : for example-the
characteristic flame colourations of metals frequently encountered in simple
organic compounds such as : Na-yellow, Ca-brick-red ; Ba-apple-green. This
forms the fundamental basis of initially called Flame Photometry, but more recently known as Flame Emission Spectroscopy (FES).
It is quite evident that a relatively large proportion of
the gaseous metal atoms shall remain in the ground state i.e., in an unexcited form. It has been observed that such
ground-state atoms shall absorb radiant energy pertaining to their own
particular resource wavelength. Therefore, when a light having the same
resonance wavelength is made to pass through a flame consisting of such atoms,
a portion of the light shall be absorbed accordingly. Furthermore, the extent
or degree of absorption would be directly proportional to the total number of
ground-state present in the flame. And this is the basis of Atomic Absorption Spectroscopy (AAS).
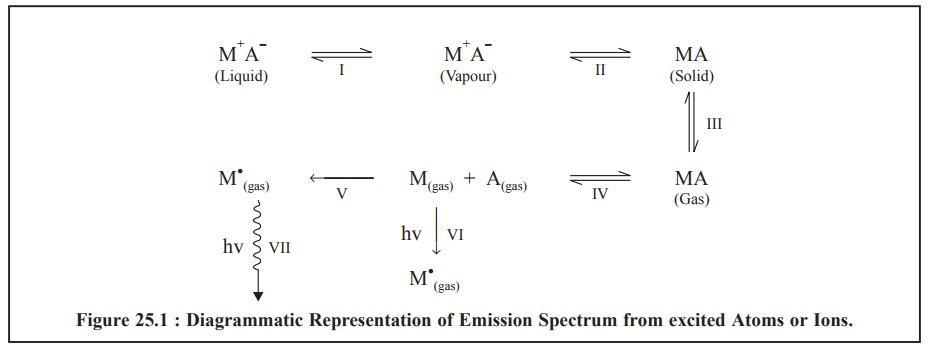
The emission spectrum thus obtained is made up of a
number of lines that actually originate from the resulting excited atoms or
ions ; and these steps may be shown diagrammatically as represented in Figure
25.1.
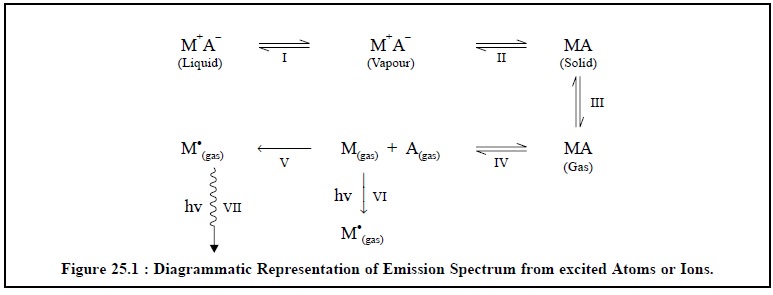
The various steps (I to
VII) in Figure 25.1, above are explained as under :
Step-I : The liquid sample containing a suitable
compound of the metal (M+ A–) is aspirated into a flame, thereby converting it
into its vapours or liquid droplets,
Step-II : The evaporation of vapours (or droplets) give
rise to the corresponding solid residue,
Step-III : The vapourization of the solid residue into
its gaseous state occurs,
Step-IV : The dissociation of the gaseous state into
its constituent atoms, namely : M(gas)+ A(gas)
take place, that initially, is in ground state,
Step-V :
The thermal excitation of some atoms into their respective higher energy levels
will lead ultimately to a condition whereby they radiate energy (flame
emission) measured by Flame Emission Spectroscopy (FES), and
Step-VI :
The absorption of radiant energy by some atoms into their higher energy levels
enable them to radiate energy (atomic absorption) measured by Atomic Absorption
Spectroscopy (AAS).
Related Topics