Chapter: 6th Science : Term 2 Unit 4 : Air
Experimental verification of presence of Oxygen, Carbon-di-oxide and Nitrogen in Air
Experimental
verification of presence of Oxygen, Carbon-di-oxide and Nitrogen in Air
Is
air a thing or a composite mixture?
For long time, that is, until eighteenth
century, human thought ‘air’ as a fundamental constituent of matter. However an
ingenious experiment conducted by Joseph Priestley in 1774 showed that "air
is not an elementary substance, but a composition," or mixture of
gases. He was also able to identify a colourless and highly reactive gas
which was later named ‘oxygen’ by the great French chemist Antoine
Lavoisier.
Priestley took a
tub of water and made a float and placed a candle on it. He covered the candle
with a glass jar.
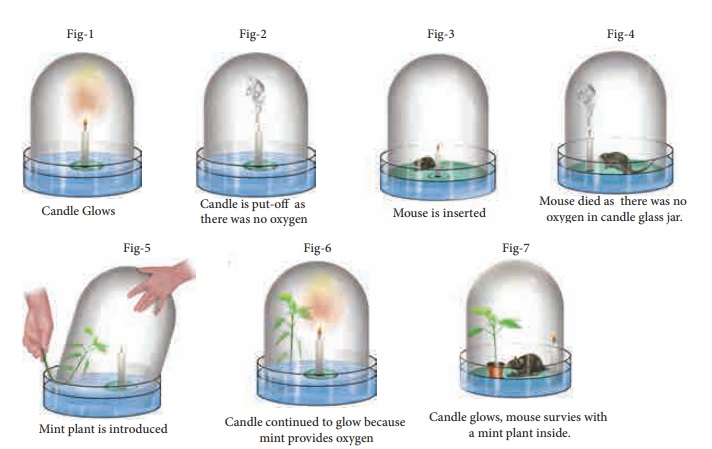
[As the bottom
portion of the jar was filled with water, no air can enter or exit and hence
the jar was completely sealed (Fig-1)]. As you would have guessed the
candle flame was extinguished in a very short time. He used a magnifying glass
to focus the sun rays to light the candle. Thus he tried to to relight the
candle many times without opening the sealed jar (Fig-2). The candle
could not be relit. What can we make out of it?
It was clear that something
in the air was being used for burning and being converted into another
substance. Once the substance in the air that was aiding the burning was
completely used by the burning flame and converted into another substance, the
flame went out.
[Later chemist named the substance necessary for burning as oxygen and during the process of burning oxygen is converted mostly into carbon dioxide.]
Now as the jar was
inside the water, Priestley could gently lift the jar and place a live mouse
inside it without allowing outside air to enter the jar (Fig-3). Without
oxygen, as you would have guessed, the mouse
died (Fig-4). It was clear that oxygen was necessary for the survival of
the mouse.
In the next step, he gently lifted the jar and
placed a mint plant (Fig-5). (Note: Look at the Figure- 5; you
could see that the plant is inserted into the bell jar when the jar is very
much inside the water. This is done to ensure that the outside air is not
entering into the bell jar.) Plant being a living thing like mouse, perhaps he
thought, would die.
Instead, the plant survived. After placing the mint plant, he lit up the candle and it
continued to burn (Fig-6).
In fourth experiment,
he took a jar, burned a candle and converted all oxygen into carbon dioxide. He
placed a mint plant and a mouse into this jar. Both the plant and the mouse
survived (Fig-7). He found that plants and animals have a synergy.
Animals consume oxygen and release carbon-di-oxide and plants take up carbon
dioxide and release oxygen.
During 1730 – 1799, Jan Ingenhousz showed that
sunlight is essential to the plant to carry out photosynthesis and also to
purify air that is fouled by breathing animals or by burning candles.
From these experiments it was clear that
“air” was a composite mixture of many gases like oxygen and carbon-di-oxide.
Proof for release of oxygen in photosynthesis
Activity
2: Take a healthy branch of Hydrilla
and place it in a funnel. Invert the funnel in a beaker of water as shown in
the figure. Invert a test tube over the stem of the funnel. The stem of the
funnel should be kept immersed inside the water.
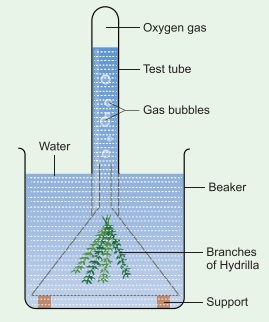
Leave
the beaker in sunlight for some time. You will notice some bubbles rising in
the test tube. The bubbles contain oxygen released by the plant during
photosynthesis. If we show a glowing splinter to the collected air, it burns
brightly. This shows that the collected gas is oxygen.
Test for the proportion of Oxygen and Nitrogen in air
Activity
3: We know that iron undergoes
rusting with oxygen and forms iron oxide. This process can be used to estimate
the percentage of oxygen in air, which has been removed by the rusting
reaction.
Take
a small portion of iron wool, press it into a 20 ml graduated test tube and wet
it with water. Tip away excess of water. Take a 500ml beaker and fill half of
the beaker with water. Invert the test tube and place it in air. Leave the
arrangement at least for a week without making any disturbance to the test
tube.
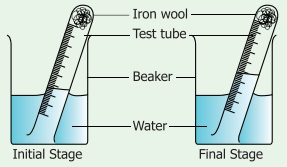
Observe
the changes that had happened in the iron wool and to the level of water inside
the test tube. We could see that the water level has increased inside the test
tube. The rise in water is because of oxygen in air which has been removed by
the rusting reaction.This will be about 20% which is approximately the
percentage of oxygen in the air.
More to Know!
Daniel Rutherford, a Scottish chemist,
discovered nitrogen. He removed oxygen and converted it into carbon-di-oxide
using an inverted bell jar using a burning candle. He passed this air without
oxygen through lime water and removed carbon-di-oxide also.
Once the
carbon-di-oxide was removed in that air, neither a candle burned nor a plant
breathed. Hence he was sure that the remaining air he had did not have oxygen
and carbon-di-oxide. He was able to produce a gas, which showed the same
property of the air without oxygen and carbon-di-oxide. Hence this gas was
named ‘nitrogen’.
Test for Carbon-di-oxide in air
Pour some lime
water in a glass tumbler. Bubble some air using a straw through the

limewater. After a few minutes, look at the
lime water carefully. The lime water will produce a white precipitate and that
the lime water will eventually turn to a milky white solution. This shows
the presence of carbon-di-oxide in air.
Related Topics