Chapter: Introduction to Human Nutrition: Nutrition Research Methodology
Epidemiological designs - Nutrition Research Methodology
Epidemiological designs
Epidemiology is a health-related science dealing with the distribution and determinants of health and illness in populations. Nutritional epidemiology inte-grates the knowledge derived from nutrition research, to examine diet–disease relationships at the level of free-living populations. Nutritional epidemiology provides scientific evidence to understand the role of nutrition in the cause and prevention of disease.
The comparison and choice of different epidemio-logical study designs depends on exposure measures, outcome measures, costs, and expected length of follow-up. The selection of a study method is often influenced by pragmatic issues such as feasibility, as well as by ethical questions.
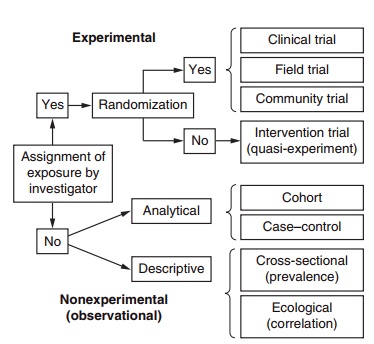
Epidemiological studies can be divided into two broad categories (Figure 13.2): experimental and nonexperimental (observational) studies. Observa-tional studies can be further divided into descriptive and analytical studies. In a wide sense, an experiment is a set of observations, conducted under controlled
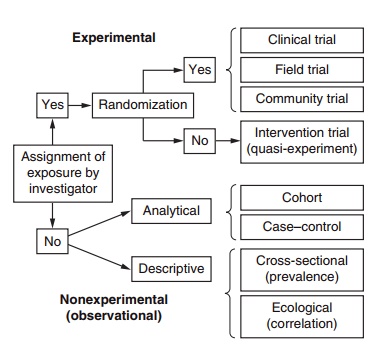
Figure 13.2 Classification of epidemiological designs
Experimental studies in nutritional epidemiology
It is necessary to consider that in biological experi-mentation, it is not possible for the scientist to control completely all of the relevant circumstances, and the manipulation will consist of increasing at most the degree of variation in the factor that the scientist is investigating. The ideal will be to obtain two almost identical sets of circumstances where all factors are the same. If a strong variation is then introduced in only one of these factors, all of the observed differ-ences between the two sets that occurred thereafter would be causally attributed to the single factor that the investigator had manipulated.
Experimental epidemiological designs are those in which the investigator assigns the exposure to each subject. In these studies, the treatment (or exposure) is assigned with the aim of attaining maximum com-parability between treated and untreated groups regarding all other characteristics of the subjects apart from the treatment or exposure of interest. In epide-miological research, the best way to achieve identical sets of circumstances is to assign subjects randomly to exposure (treatment) or control groups. This process is called randomization. All randomized studies are experimental designs.
Exposure, from an epidemiological point of view, describes lifestyle or environmental factors that may be relevant to health. Outcome is another generic term used to describe the health-related events or variables that are being studied in relation to the effect of an exposure. In nutritional epidemiology, the primary exposure of interest is dietary intake, whereas outcome measures usually involve disease occurrence or nutritional status indicators (anthropometry, clinical signs of disease/health status, biological or physiological measures or dietary habits).
It is also possible to design experimental studies assigning whole population groups to different expo-sures. These studies are called community trials. For example, if a whole town is assigned to receive an educational program about healthy eating and another neighboring town is assigned to control (no educational program), this would be a community trial; when randomization is used, it is termed “cluster- randomization.” However, when the number of ran-domized units is scarce, even though each unit may be large, there would be no guarantee that the groups to be compared would be identical. Conversely, if the randomization has been done on an individual basis and the whole sample is large enough, a random scheme will usually accomplish its objective of dis-tributing the participants in groups that are essen-tially homogeneous in all measured and unmeasured factors. This balance makes groups directly compa-rable and ensures the validity of causal inferences extracted from a randomized design (individual randomization).
In general, experimental studies with individual randomization provide the strongest evidence for the effect of an exposure on an outcome. Experimental studies are the inferentially strongest designs to demonstrate causality, but they may raise substantial ethical problems because the scheme of random assignment is used to help not the subject, but the experiment. Subjects are exposed only to meet the needs of the protocol of the study and not the indi-vidual needs of the participant. Therefore, random-ized experiments with humans can only be conducted under strict ethical conditions. It is not permissible to carry out experimental studies where the exposure is potentially harmful. Therefore, under these conditions, nonexperimental (observational) study designs must be applied. The design options in nutritional epidemiology must take into account the setting, uses, advantages, and limita-tions (Table 13.7).
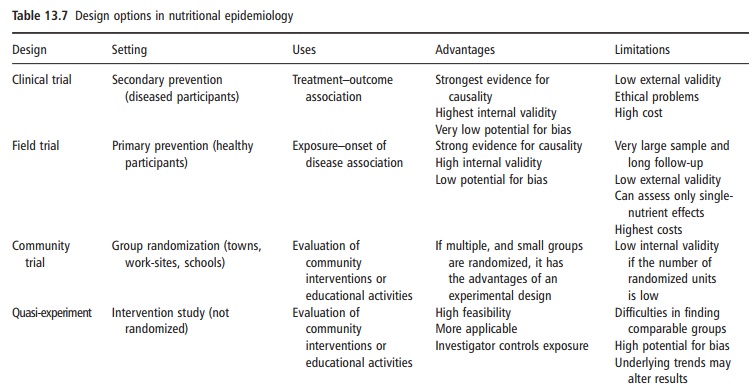
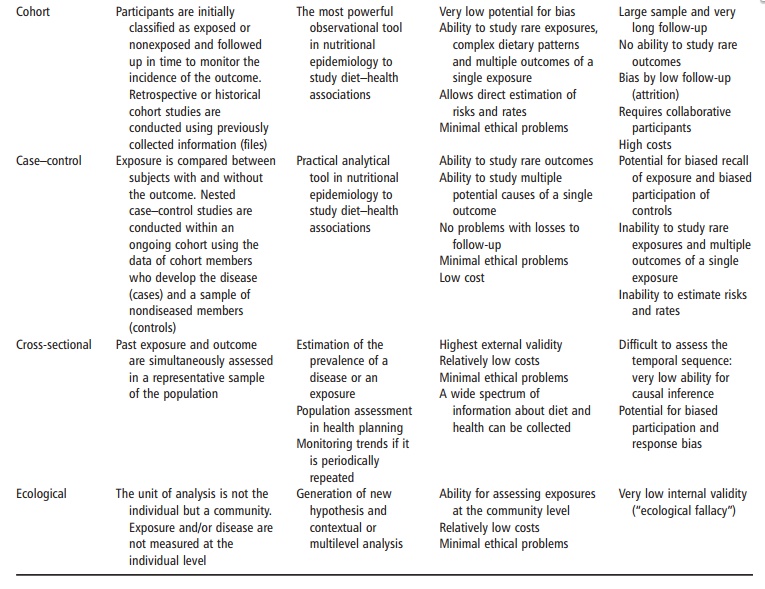
Experimental designs in epidemiology
Experimental epidemiologists try to conduct con-trolled studies, and in these studies it is the investiga-tor who assigns the exposure. Human studies, however, unlike animal studies, involve aspects that the inves-tigator cannot control. This is particularly so when they are carried out on a free-living population. Two study designs dominate this area of epidemiology: randomized controlled trials and crossover studies. In these studies, subjects are randomly assigned to
The placebo is a substance that is indistinguishable from the treatment and enables both subjects and investigators to be blinded to the treatment. Changes in indicators of health or disease status are compared between the two groups at the end of the experiment to identify the effect of the exposure.
Crossover designs in epidemiology operate on the same principles as the repeated-measures designs common to basic science research. All study subjects receive the treatment and the placebo for equal periods, with a washout period in between, and the order of treatment or placebo administration is selected at random for each study subject. Crossover designs are appropriate only for studies of treatments that have no lasting effects, a feature that limits their utility in nutritional epidemiology.
In general, experimental epidemiological study designs are well suited to the identification of causal relationships between specific exposures and indica-tors of health or disease status. Application of these methods is limited, however, by the difficulty in con-trolling exposures and by the enormous expense associated with population-based intervention trials aimed at modifying risk or chronic diseases. It is perhaps more feasible to apply experimental study designs to contrast the effects of pharmacological doses of specific nutrients or food components the exposures of which can be more easily controlled. This approach has been increasingly selected from the 1990s to assess the effects of specific micronutrients (β-carotene, α-tocopherol, folic acid, and other minerals and vitamins) using large-scale randomized trials.
When only one micronutrient is compared with a placebo, the study is called a single trial, whereas mul-tiple or factorial trials involve designs where several micronutrients are compared with a placebo. In a 2 × 2 factorial design, two treatments are evaluated simultaneously by forming four groups (treatment A, treatment B, both treatments, and placebo).
Experimental studies keep the highest internal validity among epidemiological designs. However, they may lack generalization (i.e., they may have low external validity) and their applicability to free-living populations may be poor insofar as the dietary intake patterns do not correspond to isolated nutrients but to the combination of more complex food items.
Moreover, the induction time needed to appraise the effect of a postulated cause may last longer than the observation period of a randomized trial, thus pre-cluding the ability of the trial to ascertain the causal relationship.
Quasi-experimental studies are those in which the assignment of exposure is controlled by the investigator, but subjects are not randomly allocated. They are sometimes called intervention trials (Figure 13.2).
Some randomized trials are referred to as primary prevention trials and others as secondary prevention trials. Primary prevention trials are those conducted among healthy individuals with the aim of preventing the onset of disease. For example, in the Women’s Health Initiative (Howard et al., 2006) more than 48 000 healthy postmenopausal women were ran-domly assigned to receive either a low-fat diet or placebo to prevent the onset of cardiovascular disease (CVD). All participants were free of this disease at the start of the study and they were followed up for several years to assess the incidence of fatal and non-fatal coronary heart disease, fatal and nonfatal stroke, and CVD (composite of both). This is an example of a primary prevention trial. Primary prevention trials are also called field trials.
Secondary prevention trials are conducted among patients who already suffer from a particular disease and they are randomly assigned to treatment or placebo groups to prevent adverse outcomes. For example, to study the benefits of a Mediterranean-style diet, in the Lyon Diet Heart Study, patients were randomized to two different dietary patterns after suffering a myocardial infarction (de Lorgeril et al., 1999). The outcome was not the onset of disease but the incidence of reinfarction or cardiac death during the follow-up period.
Nonexperimental (observational) epidemiological studies
When experiments are not feasible or are unethical, other nonexperimental designs are used. In nonex-perimental (observational) studies the investigator has no control over the exposure, because the participants freely assign themselves or not to the exposure. In nonexperimental studies the investigator may take advantage of “natural experiments,” where exposure only appears in some defined groups. An example of this would be an “experiment” where dietary intake is culturally determined, such as in Indonesia, where the rice consumed is white rather than brown, and beriberi is common as a result of vitamin B1 deficiency.
Nonexperimental (observational) designs can be further classified into four main subtypes:
● cross-sectional studies
● case–control studies
● cohort studies
● ecological studies.
Among observational studies, the main differences between study designs relate to the time when expo-sure and outcome are measured. The initiative “STrengthening the Reporting of OBservational studies in Epidemiology (STROBE), http://www. strobe-statement.org” provides a check-list to assess the methodological quality of the three major epide-miological designs: cohort studies, case–control studies, and cross-sectional studies (Von Elm and Egger 2004).
Cross-sectional (prevalence) studies
Cross-sectional or prevalence studies measure both exposure and outcome in the present and at the same point in time. Cross-sectional surveys provide a snap-shot of descriptive epidemiological data on nutrition, identifying nutritional needs in the population and forming a basis for health promotion and disease pre-vention programs at a single point in time. Several countries conduct regular cross-sectional surveys on representative samples of their populations focusing on dietary habits and frequencies of illness. Dietary factors can then be correlated with prevalence of illness, which may be helpful for national nutrition policy.
Case–control studies
In case–control studies, outcome is measured in the present, and past exposure is ascertained. Usually the dietary and lifestyle patterns of patients with a disease (cases) are compared with those of age- and gender-matched people without disease (controls).
Subjects are identified and recruited on the basis of the presence or absence of the disease or the health outcome variable of interest. Ideally, the controls are randomly selected from the same study base as the cases, and identical inclusion and exclusion criteria are applied to each group. The presence of specific dietary exposures or other factors of etiological interest in subjects is generally established using interviews, questionnaires, or medical record reviews. Within the general framework for case–control studies, there are several options for study design and control selection.
For example, controls may be matched with cases at an individual level on the basis of age, gender, or other variables believed to affect disease risk. Match-ing eliminates variability between cases and controls with respect to the matching variables and thus leads to a higher efficiency in the analysis. Nevertheless, matching does not control for the confounding effects of these risk factors on the observed relationship. Case–control studies are by far the most logistically feasible of the analytical study designs in epidemiol-ogy, but their application to questions of interest to nutritionists is limited by the particular nature of diet–disease relationships.
The insight to be gained from a comparison of dietary exposures between cases and controls is limited by the possibility that the dietary patterns of subjects have changed since the time when diet was most important to the disease initiation process. Ret-rospective case–control studies attempt to overcome this limitation by measuring past diet using food fre-quency or diet history methods. One concern is that recall of past diet by cases may be influenced by their present disease status. For example, patients who have had a heart attack may attach an unfair level of impor-tance to their intake of specific foods, based on misinformation.
A primary factor in choosing between a case– control design and a cohort design is whether the exposure or the outcome is rare. If the outcome is rare, case–control studies are preferable, because a cohort would need a very large sample to observe a sufficient number of events. If the exposure is rare, cohort studies are preferable.
A nested case–control design consists of selecting as cases only those members of a previously defined cohort who develop the disease during their follow-up period. A random sample or a matched sample of non-cases is also selected from the cohort to make up the control series as the comparison group.
Cohort studies
In cohort studies exposure is evaluated in the present and outcome ascertained in the future. Cohort studies are most commonly longitudinal or prospective, with subjects being followed forward in time over some predefined period to assess disease onset. They may also be retrospective (historical cohorts), with groups identified on the basis of expo-sure sometime in the past and then followed from that time to the present to establish presence or absence of the outcome. The feasibility of retrospective cohorts depends on the availability of good-quality data from pre-existing files. The research costs associated with cohort study designs mean that such studies are less common than other approaches. Nevertheless, a sub-stantial effort to develop large cohort studies in nutri-tional epidemiology has been made since the early 1980s. Cohort studies can assess multiple outcomes, whereas case–control studies are restricted to assess-ing one outcome, but may be able to assess many dif-ferent exposures. If an absolute measure of the effect of the exposure on the outcome is required, the only design that is appropriate is a cohort study, as case– control studies cannot be used to estimate incidence.
For example, to ascertain the relationship between olive oil consumption and coronary heart disease, a case–control study would compare the previous con-sumption of olive oil between cases of myocardial infarction and healthy controls. A cohort study would start with a roster of healthy individuals whose base-line diet would be recorded. They would then be fol-lowed up over several years to compare the occurrence of new cases of myocardial infarction between those consuming different levels of olive oil as recorded when they were healthy at baseline.
Ecological studies
Epidemiological studies can be classified according to whether measurements of exposure and outcome are made on populations or individuals. Observational investigations in which the unit of observation and analysis is not the individual but a whole community or population are called ecological studies. In ecologi-cal studies, measures of exposure routinely collected and aggregated at the household, local, district, regional, national, or international level are compared with outcome measures aggregated at the same level. An example of an ecological study would be plotting the mortality rates for colon cancer in several coun-tries against the average intakes of saturated fat in these same countries and calculating the correlation between the two variables.
Studies considering the individual (instead of the population) as the unit of observation are always preferable because in an individually based study it is possible to relate exposure and outcome measures more directly, preventing many flaws that are likely to invalidate the findings of ecological studies. One of these flaws is known as the “ecological fallacy” and it is the bias resulting because an association observed between variables on an aggregated level does not necessarily represent the association that exists at an individual level. A major advantage of individually based studies over aggregated studies is that they allow the direct estimation of the risk of disease in relation to exposure.
Ecological studies measure diet less accurately because they use the average population intake as the exposure value for all individuals in the groups that are compared, leading to a high potential for biased ascertainment of diet–disease associations. Ecological studies, also termed correlation studies, may compare indicators of diet and health or disease within a single population over time to look for secular trends, or to compare the disease incidence rates and dietary intake patterns of migrant groups with those of comparable populations in the original and new country. Ecological comparisons have been important in hypothesizing diet and disease associa-tions. Nevertheless, they are not able to establish causal relationships.
Definition of outcomes and end-points
Epidemiological outcomes must be clearly defined at the outset of a study. For example, a study of diet and CVD may specify that the outcome (CVD) is verified by specific clinical tests such as cardiac enzyme level or electrocardiographic changes. Taking the word of the patient or the doctor is not sufficient. Two main measures of the frequency for an outcome are used in epidemiology: prevalence and incidence.
Prevalence
The prevalence of an outcome is the proportion of subjects in a population who have that outcome at a given point in time. The numerator of prevalence is the number of existing cases and the denominator is the whole population:
Prevalence = Existing cases/ Total population
Incidence
The incidence of an outcome is the proportion of new cases that occur in a population during a period of observation. The numerator of incidence is the number of new cases developing during the follow-up period, while the denominator is the total population at risk at the beginning of the follow-up time:
Incidence = New cases/ Population inititally at risk
When calculated in this fashion, incidence is a pro-portion. However, incidence can also be expressed as a rate (velocity or density), when the time during which each person is observed (i.e., person-time of observation) is included in the denominator. Then it is called incidence rate or incidence density and it is expressed as the number of new cases per person-time of observation.
Other epidemiological methods
Epidemiological studies have also been conducted to assess: consumer attitudes to and beliefs about food, nutrition, physical activity patterns, and health to provide policy-makers, researchers and the food industry with data to promote health messages con-cerning the relation between food or nutrient intake and chronic diseases. These surveys seek information about influences on food choice, health determinants, criteria about perceptions of healthy eating, regular sources of nutritional information, expected benefits and barriers to healthy diet implementation, in order to identify consumers’ knowledge, attitudes, and beliefs concerning food and health interactions and to promote more focused nutrition education messages.
Meta-analysis and pooled analysis
The role of meta-analysis for systematically combin-ing the results of published randomized trials has become routine, but its place in observational epide-miology has been controversial despite widespread use in social sciences. Some have argued that the com-bining of data from randomized trials is appropriate because statistical power is increased without concern for validity since the comparison groups have been randomized, but that in observational epidemiology the issue of validity is determined largely by con-founding and bias rather than limitations of statistical power. Thus, the greater statistical precision obtained by the combination of data may be misleading because the findings may still be invalid. An alternative to combining published epidemiological data is to pool and analyze the primary data from all available studies on a topic that meets specified criteria. Ideally, this should involve the active collaboration of the original investigators, who are fully familiar with the data and its limitations. This kind of study conducted with a combination of the original data from several studies, is the basis of pooled analysis or pooling projects. In a pooled analysis, the range of dietary factors that can be addressed may be considerably greater than in the separate analyses because any one study will have few subjects in the extremes of intake and, sometimes, the studies will vary in distribution of dietary factors. The advantages of pooled analyses in nutritional epidemi-ology are so substantial that they are becoming common practice for important issues, such as alco-holic intake and breast cancer, body size and breast cancer, or alcohol beverages and coronary heart disease.
Analysis of epidemiological data requires careful consideration of the criteria for acceptable data quality, but also of the presentation of categorized or continuous independent variables and the applica-tion of empirical scores. The study of subgroup analy-sis and interactions and error correction are other issues of interest. Other limitations are the require-ment of sample to be considered as representative, compliance, inaccuracies of information in retrospec-tive studies, and confounding effects by factors that are simultaneously associated with both the exposure and the outcome.
Related Topics